Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
REVIEW <strong>Palladium</strong>- <strong>and</strong> <strong>Copper</strong>-<strong>Catalyzed</strong> Heterocycle Synthesis 7<br />
helicenes. 43b The second example shown in Scheme 15<br />
was reported by Chida <strong>and</strong> colleagues, <strong>and</strong> shows that related<br />
strategies developed using dibromobiphenyls are<br />
also effective in carbazole synthesis. 44a The example<br />
shows the preparation of a key intermediate in the synthesis<br />
of the natural product murrayazoline. 44b In order to<br />
achieve an efficient transformation using the sterically dem<strong>and</strong>ing<br />
amine 39, it was necessary to employ a high catalyst<br />
loading (20 mol%). A copper-catalyzed version of<br />
these transformations has also been reported. 45 The cascade<br />
coupling of nitrogen nucleophiles with doubly activated<br />
bi-aromatics has proven to be a powerful method for<br />
carbazole synthesis <strong>and</strong> has been exploited by several other<br />
groups. For example, Samyn <strong>and</strong> co-workers utilized<br />
dibromo-2,2¢-bithiophene substrates to prepare<br />
dithienopyrroles, 46 as did the Barlow research group. 47<br />
MeO<br />
Me<br />
O<br />
37<br />
OMOM<br />
Scheme 15<br />
O<br />
OMe<br />
OTf<br />
OTf<br />
Pd2(dba)3.CHCl3<br />
(5 mol%)<br />
XantPhos (2) (10 mol%)<br />
K3PO4<br />
o-xylene, 100 °C<br />
Br<br />
Pd2(dba) 3 (20 mol%)<br />
XPhos (32) (60 mol%)<br />
Br<br />
H2N +<br />
Me<br />
Me<br />
NaOt-Bu<br />
toluene, 130 °C<br />
O<br />
+<br />
t-BuO<br />
NH2<br />
39<br />
O<br />
MeO2C<br />
Bedford et al. developed a cascade sequence based on intermolecular<br />
aryl-amination followed by intramolecular<br />
direct C–H arylation as a route to carbazole derivatives;<br />
several examples are shown in Scheme 16. 48 The process<br />
combines o-chloroanilines with aryl bromides using palladium<br />
catalysis. Use of the bulky electron-rich tri(tertbutyl)phosphine<br />
lig<strong>and</strong> in combination with palladium(II)<br />
acetate was the optimal catalyst system <strong>and</strong> allowed the<br />
preparation of a range of carbazoles in good yields. Significant<br />
substitution could be tolerated on the coupling<br />
partners, as illustrated by the preparation of the natural<br />
product clausine P (40).<br />
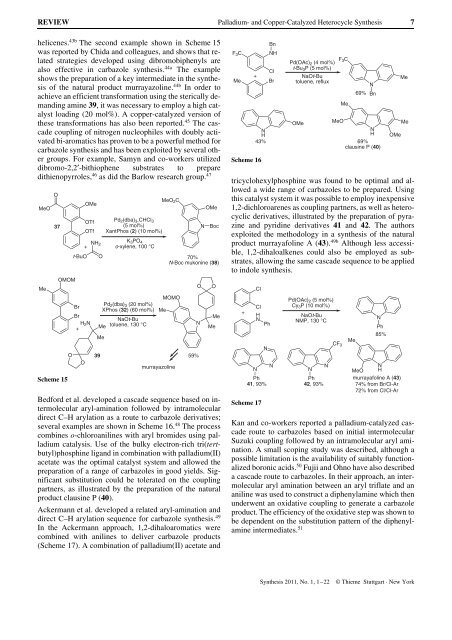
Ackermann et al. developed a related aryl-amination <strong>and</strong><br />
direct C–H arylation sequence for carbazole synthesis. 49<br />
In the Ackermann approach, 1,2-dihaloaromatics were<br />
combined with anilines to deliver carbazole products<br />
(Scheme 17). A combination of palladium(II) acetate <strong>and</strong><br />
Me<br />
N<br />
OMe<br />
Boc<br />
70%<br />
N-Boc mukonine (38)<br />
MOMO<br />
murrayazoline<br />
59%<br />
O O<br />
Me<br />
N<br />
Me<br />
F 3C<br />
Me<br />
Scheme 16<br />
tricyclohexylphosphine was found to be optimal <strong>and</strong> allowed<br />
a wide range of carbazoles to be prepared. Using<br />
this catalyst system it was possible to employ inexpensive<br />
1,2-dichloroarenes as coupling partners, as well as heterocyclic<br />
derivatives, illustrated by the preparation of pyrazine<br />
<strong>and</strong> pyridine derivatives 41 <strong>and</strong> 42. The authors<br />
exploited the methodology in a synthesis of the natural<br />
product murrayafoline A (43). 49b Although less accessible,<br />
1,2-dihaloalkenes could also be employed as substrates,<br />
allowing the same cascade sequence to be applied<br />
to indole synthesis.<br />
+<br />
+<br />
N<br />
H<br />
43%<br />
Cl<br />
H<br />
N<br />
N<br />
Cl<br />
Scheme 17<br />
Ph<br />
N<br />
Ph<br />
41, 93%<br />
Bn<br />
NH<br />
Cl<br />
Br<br />
N<br />
Pd(OAc)2 (4 mol%)<br />
t-Bu3P (5 mol%)<br />
NaOt-Bu<br />
toluene, reflux<br />
OMe<br />
Pd(OAc)2 (5 mol%)<br />
Cy3P (10 mol%)<br />
NaOt-Bu<br />
NMP, 130 °C<br />
N<br />
Ph<br />
42, 93%<br />
N<br />
69% Bn<br />
Kan <strong>and</strong> co-workers reported a palladium-catalyzed cascade<br />
route to carbazoles based on initial intermolecular<br />
Suzuki coupling followed by an intramolecular aryl amination.<br />
A small scoping study was described, although a<br />
possible limitation is the availability of suitably functionalized<br />
boronic acids. 50 Fujii <strong>and</strong> Ohno have also described<br />
a cascade route to carbazoles. In their approach, an intermolecular<br />
aryl amination between an aryl triflate <strong>and</strong> an<br />
aniline was used to construct a diphenylamine which then<br />
underwent an oxidative coupling to generate a carbazole<br />
product. The efficiency of the oxidative step was shown to<br />
be dependent on the substitution pattern of the diphenylamine<br />
intermediates. 51<br />
N<br />
F 3C<br />
MeO<br />
N<br />
H<br />
69%<br />
clausine P (40)<br />
CF 3<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
Me<br />
Me<br />
N<br />
Ph<br />
85%<br />
OMe<br />
N<br />
MeO H<br />
murrayafoline A (43)<br />
74% from Br/Cl-Ar<br />
72% from Cl/Cl-Ar<br />
Me<br />
Me