Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
REVIEW <strong>Palladium</strong>- <strong>and</strong> <strong>Copper</strong>-<strong>Catalyzed</strong> Heterocycle Synthesis 5<br />
Br OTf<br />
Cl<br />
+<br />
20 H2N<br />
N<br />
71%<br />
Ph<br />
Scheme 8<br />
7-azaindole in good yield (Scheme 9). The process was<br />
shown to work well for 7-azaindoles; however, access to<br />
the remaining regioisomers was less successful.<br />
Scheme 9<br />
The 2-(2-haloalkenyl)aryl halide substrates have also<br />
been shown to undergo cascade copper-catalyzed amination<br />
reactions to deliver N-functionalized indoles. 35f An<br />
example featuring a carbamate coupling partner is shown<br />
in Scheme 10. Although some overlap in scope with the<br />
palladium-catalyzed version of the process was established,<br />
there were also significant differences in reactivity.<br />
In general, the copper-catalyzed variant was more<br />
limited, with chloro-substituted substrates performing<br />
poorly; however, greater success was achieved with<br />
amide nucleophiles, relative to the palladium system.<br />
Scheme 10<br />
Pd2(dba)3 (2.5 mol%)<br />
DPEPhos (6 mol%)<br />
Cs2CO3 toluene, 100 °C<br />
Br Cl<br />
Ph<br />
21, E/Z 2:7<br />
+ H2N<br />
Ph<br />
Pd2(dba)3 (2.5 mol%)<br />
SPhos (14) (7.5 mol%)<br />
NaOt-Bu<br />
toluene, 80 °C<br />
N Cl Br<br />
+<br />
O<br />
OEt<br />
24<br />
H2N OMe<br />
O<br />
PPh2 PPh2<br />
DPEPhos (22)<br />
N<br />
Ph 81%<br />
OMe<br />
Pd2(dba) 3 (5 mol%)<br />
DPEPhos (22) (12 mol%)<br />
Cs2CO3 toluene, 110 °C<br />
Br Br<br />
25<br />
MeO<br />
CuOAc (10 mol%)<br />
MeO<br />
MeO<br />
H2N<br />
+<br />
Ot-Bu<br />
(20 mol%)<br />
Cs2CO3<br />
toluene, 110 °C<br />
MeO<br />
O<br />
MeN NMe<br />
H H 25<br />
75%<br />
94%<br />
N<br />
N<br />
Ph<br />
N<br />
23, 77%<br />
Me Me<br />
N<br />
83%<br />
N<br />
t-BuO<br />
82%<br />
OEt<br />
O<br />
OMe<br />
N<br />
O<br />
Cl<br />
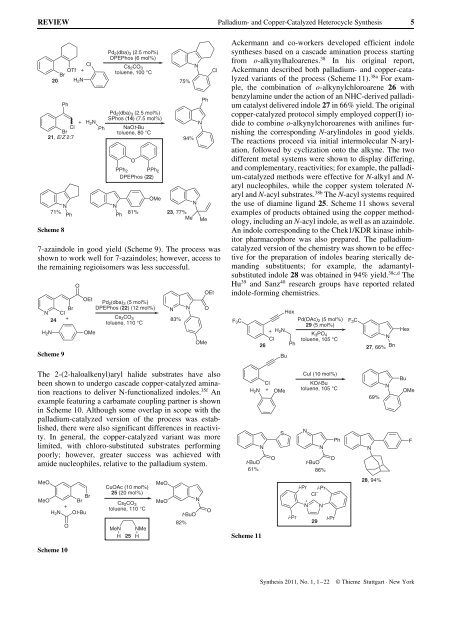
Ackermann <strong>and</strong> co-workers developed efficient indole<br />
syntheses based on a cascade amination process starting<br />
from o-alkynylhaloarenes. 38 In his original report,<br />
Ackermann described both palladium- <strong>and</strong> copper-catalyzed<br />
variants of the process (Scheme 11). 38a For example,<br />
the combination of o-alkynylchloroarene 26 with<br />
benzylamine under the action of an NHC-derived palladium<br />
catalyst delivered indole 27 in 66% yield. The original<br />
copper-catalyzed protocol simply employed copper(I) iodide<br />
to combine o-alkynylchoroarenes with anilines furnishing<br />
the corresponding N-arylindoles in good yields.<br />
The reactions proceed via initial intermolecular N-arylation,<br />
followed by cyclization onto the alkyne. The two<br />
different metal systems were shown to display differing,<br />
<strong>and</strong> complementary, reactivities; for example, the palladium-catalyzed<br />
methods were effective for N-alkyl <strong>and</strong> Naryl<br />
nucleophiles, while the copper system tolerated Naryl<br />
<strong>and</strong> N-acyl substrates. 38b The N-acyl systems required<br />
the use of diamine lig<strong>and</strong> 25. Scheme 11 shows several<br />
examples of products obtained using the copper methodology,<br />
including an N-acyl indole, as well as an azaindole.<br />
An indole corresponding to the Chek1/KDR kinase inhibitor<br />
pharmacophore was also prepared. The palladiumcatalyzed<br />
version of the chemistry was shown to be effective<br />
for the preparation of indoles bearing sterically dem<strong>and</strong>ing<br />
substituents; for example, the adamantylsubstituted<br />
indole 28 was obtained in 94% yield. 38c,d The<br />
Hu 39 <strong>and</strong> Sanz 40 research groups have reported related<br />
indole-forming chemistries.<br />
F3C<br />
Scheme 11<br />
29<br />
26<br />
Hex<br />
Pd(OAc)2 (5 mol%)<br />
(5 mol%)<br />
+<br />
H2N<br />
Cl<br />
Ph<br />
K3PO4<br />
toluene, 105 °C<br />
Bu<br />
i-Pr i-Pr<br />
Cl<br />
+<br />
N N<br />
–<br />
F 3C<br />
N<br />
27, 66% Bn<br />
Hex<br />
H2N<br />
Cl<br />
+ OMe<br />
CuI (10 mol%)<br />
KOt-Bu<br />
toluene, 105 °C<br />
N<br />
Bu<br />
OMe<br />
69%<br />
t-BuO<br />
61%<br />
N<br />
O<br />
S<br />
N<br />
N<br />
t-BuO<br />
86%<br />
O<br />
i-Pr i-Pr<br />
29<br />
Ph<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
N<br />
28, 94%<br />
F