Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
Palladium- and Copper-Catalyzed Aryl Halide Amination ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
REVIEW <strong>Palladium</strong>- <strong>and</strong> <strong>Copper</strong>-<strong>Catalyzed</strong> Heterocycle Synthesis 3<br />
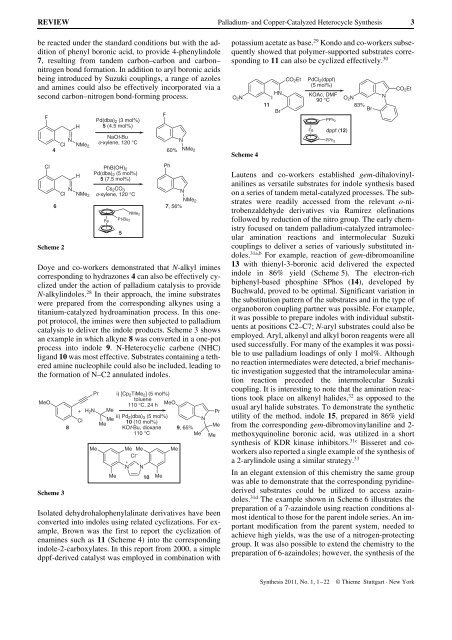
be reacted under the st<strong>and</strong>ard conditions but with the addition<br />
of phenyl boronic acid, to provide 4-phenylindole<br />
7, resulting from t<strong>and</strong>em carbon–carbon <strong>and</strong> carbon–<br />
nitrogen bond formation. In addition to aryl boronic acids<br />
being introduced by Suzuki couplings, a range of azoles<br />
<strong>and</strong> amines could also be effectively incorporated via a<br />
second carbon–nitrogen bond-forming process.<br />
F<br />
Cl<br />
4<br />
6<br />
Scheme 2<br />
Doye <strong>and</strong> co-workers demonstrated that N-alkyl imines<br />
corresponding to hydrazones 4 can also be effectively cyclized<br />
under the action of palladium catalysis to provide<br />
N-alkylindoles. 28 In their approach, the imine substrates<br />
were prepared from the corresponding alkynes using a<br />
titanium-catalyzed hydroamination process. In this onepot<br />
protocol, the imines were then subjected to palladium<br />
catalysis to deliver the indole products. Scheme 3 shows<br />
an example in which alkyne 8 was converted in a one-pot<br />
process into indole 9. N-Heterocyclic carbene (NHC)<br />
lig<strong>and</strong> 10 was most effective. Substrates containing a tethered<br />
amine nucleophile could also be included, leading to<br />
the formation of N–C2 annulated indoles.<br />
Scheme 3<br />
H<br />
N<br />
Cl NMe2<br />
H<br />
N<br />
Cl NMe2<br />
Pd(dba) 2 (3 mol%)<br />
5 (4.5 mol%)<br />
NaOt-Bu<br />
o-xylene, 120 °C<br />
PhB(OH) 2<br />
Pd(dba)2 (5 mol%)<br />
5 (7.5 mol%)<br />
Cs 2CO 3<br />
o-xylene, 120 °C<br />
Fe<br />
NMe2 Pt-Bu2<br />
5<br />
N<br />
60% NMe2 N<br />
NMe2 7, 56%<br />
10<br />
8<br />
MeO<br />
Pr i) [Cp2TiMe2] (5 mol%)<br />
toluene<br />
110 °C, 24 h<br />
MeO<br />
+ H2N Me<br />
ii) Pd2(dba)3 (5 mol%)<br />
Cl Me<br />
Me<br />
(10 mol%)<br />
KOt-Bu, dioxane 9, 65%<br />
N<br />
Pr<br />
Me<br />
110 °C<br />
Me Me<br />
Me<br />
Me<br />
Isolated dehydrohalophenylalinate derivatives have been<br />
converted into indoles using related cyclizations. For example,<br />
Brown was the first to report the cyclization of<br />
enamines such as 11 (Scheme 4) into the corresponding<br />
indole-2-carboxylates. In this report from 2000, a simple<br />
dppf-derived catalyst was employed in combination with<br />
F<br />
Ph<br />
Me Me Me<br />
Cl<br />
N N<br />
Me<br />
–<br />
10<br />
+<br />
potassium acetate as base. 29 Kondo <strong>and</strong> co-workers subsequently<br />
showed that polymer-supported substrates corresponding<br />
to 11 can also be cyclized effectively. 30<br />
O 2N<br />
Scheme 4<br />
11<br />
HN<br />
I<br />
Br<br />
CO2Et<br />
PdCl 2(dppf)<br />
(5 mol%)<br />
KOAc, DMF<br />
90 °C<br />
PPh 2<br />
Fe dppf (12)<br />
PPh2<br />
O2N 83%<br />
Br<br />
Lautens <strong>and</strong> co-workers established gem-dihalovinylanilines<br />
as versatile substrates for indole synthesis based<br />
on a series of t<strong>and</strong>em metal-catalyzed processes. The substrates<br />
were readily accessed from the relevant o-nitrobenzaldehyde<br />
derivatives via Ramirez olefinations<br />
followed by reduction of the nitro group. The early chemistry<br />
focused on t<strong>and</strong>em palladium-catalyzed intramolecular<br />
amination reactions <strong>and</strong> intermolecular Suzuki<br />
couplings to deliver a series of variously substituted indoles.<br />
31a,b For example, reaction of gem-dibromoaniline<br />
13 with thienyl-3-boronic acid delivered the expected<br />
indole in 86% yield (Scheme 5). The electron-rich<br />
biphenyl-based phosphine SPhos (14), developed by<br />
Buchwald, proved to be optimal. Significant variation in<br />
the substitution pattern of the substrates <strong>and</strong> in the type of<br />
organoboron coupling partner was possible. For example,<br />
it was possible to prepare indoles with individual substituents<br />
at positions C2–C7; N-aryl substrates could also be<br />
employed. <strong>Aryl</strong>, alkenyl <strong>and</strong> alkyl boron reagents were all<br />
used successfully. For many of the examples it was possible<br />
to use palladium loadings of only 1 mol%. Although<br />
no reaction intermediates were detected, a brief mechanistic<br />
investigation suggested that the intramolecular amination<br />
reaction preceded the intermolecular Suzuki<br />
coupling. It is interesting to note that the amination reactions<br />
took place on alkenyl halides, 32 as opposed to the<br />
usual aryl halide substrates. To demonstrate the synthetic<br />
utility of the method, indole 15, prepared in 86% yield<br />
from the corresponding gem-dibromovinylaniline <strong>and</strong> 2methoxyquinoline<br />
boronic acid, was utilized in a short<br />
synthesis of KDR kinase inhibitors. 31c Bisseret <strong>and</strong> coworkers<br />
also reported a single example of the synthesis of<br />
a 2-arylindole using a similar strategy. 33<br />
In an elegant extension of this chemistry the same group<br />
was able to demonstrate that the corresponding pyridinederived<br />
substrates could be utilized to access azaindoles.<br />
31d The example shown in Scheme 6 illustrates the<br />
preparation of a 7-azaindole using reaction conditions almost<br />
identical to those for the parent indole series. An important<br />
modification from the parent system, needed to<br />
achieve high yields, was the use of a nitrogen-protecting<br />
group. It was also possible to extend the chemistry to the<br />
preparation of 6-azaindoles; however, the synthesis of the<br />
Synthesis 2011, No. 1, 1–22 © Thieme Stuttgart · New York<br />
N<br />
CO2Et