Exploration and Optimization of Tellurium‐Based Thermoelectrics
Exploration and Optimization of Tellurium‐Based Thermoelectrics
Exploration and Optimization of Tellurium‐Based Thermoelectrics
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(AgSbTe2)1‐x(GeTe)x that has the same rock salt structure as PbTe for x > 0.8. With x < 0.8, the TAGS<br />
structure transforms into rhombohedral‐type where x = 0.7 is preferential for structure‐formation. [45]<br />
TAGS performs optimally between 0.8 < x < 0.85 [44, 46] <strong>and</strong> is a popular thermoelectric chalcogenide that<br />
has been used in several <strong>of</strong> the applications mentioned in 1.3.1.<br />
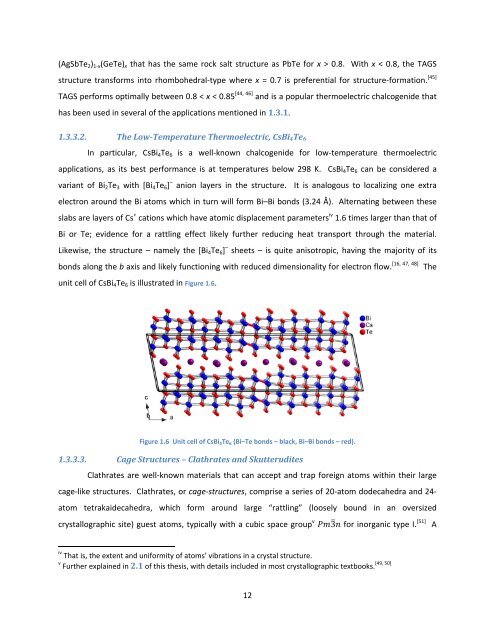
1.3.3.2. The Low‐Temperature Thermoelectric, CsBi4Te6<br />
In particular, CsBi4Te6 is a well‐known chalcogenide for low‐temperature thermoelectric<br />
applications, as its best performance is at temperatures below 298 K. CsBi4Te6 can be considered a<br />
variant <strong>of</strong> Bi2Te3 with [Bi4Te6] – anion layers in the structure. It is analogous to localizing one extra<br />
electron around the Bi atoms which in turn will form Bi–Bi bonds (3.24 Å). Alternating between these<br />
slabs are layers <strong>of</strong> Cs + cations which have atomic displacement parameters iv 1.6 times larger than that <strong>of</strong><br />
Bi or Te; evidence for a rattling effect likely further reducing heat transport through the material.<br />
Likewise, the structure – namely the [Bi4Te6] – sheets – is quite anisotropic, having the majority <strong>of</strong> its<br />
bonds along the b axis <strong>and</strong> likely functioning with reduced dimensionality for electron flow. [16, 47, 48] The<br />
unit cell <strong>of</strong> CsBi4Te6 is illustrated in Figure 1.6.<br />
Figure 1.6 Unit cell <strong>of</strong> CsBi 4Te 6 (Bi–Te bonds – black, Bi–Bi bonds – red).<br />
1.3.3.3. Cage Structures – Clathrates <strong>and</strong> Skutterudites<br />
Clathrates are well‐known materials that can accept <strong>and</strong> trap foreign atoms within their large<br />
cage‐like structures. Clathrates, or cage‐structures, comprise a series <strong>of</strong> 20‐atom dodecahedra <strong>and</strong> 24‐<br />
atom tetrakaidecahedra, which form around large “rattling” (loosely bound in an oversized<br />
crystallographic site) guest atoms, typically with a cubic space group v 3 for inorganic type I. [51] A<br />
iv That is, the extent <strong>and</strong> uniformity <strong>of</strong> atoms’ vibrations in a crystal structure.<br />
v Further explained in 2.1 <strong>of</strong> this thesis, with details included in most crystallographic textbooks. [49, 50]<br />
12