Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
4.2 Summary and critique <strong>of</strong> submitted cl<strong>in</strong>ical effectiveness evidence<br />
If <strong>the</strong>re is more than one RCT described <strong>in</strong> <strong>the</strong> MS, it may be appropriate to discuss each trial<br />
<strong>in</strong>dividually us<strong>in</strong>g <strong>the</strong> head<strong>in</strong>gs described.<br />
4.2.1 Summary <strong>of</strong> submitted cl<strong>in</strong>ical evidence <strong>for</strong> each relevant trial.<br />
The MS identified four trials <strong>of</strong> apixaban versus enoxapar<strong>in</strong> (ADVANCE-1, 14, 15 16, 17<br />
ADVANCE-2,<br />
ADVANCE-3 18, 19 and APROPOS 20 ) Results <strong>of</strong> <strong>the</strong> ADVANCE-1, -2 & -3 trials are described <strong>in</strong><br />
section 5.5 and 5.9 <strong>of</strong> <strong>the</strong> MS (MS, pages 55-68, 103-116). Accord<strong>in</strong>g to <strong>the</strong> manufacturer<br />
“APROPOS is a phase II dose f<strong>in</strong>d<strong>in</strong>g study and as such is not presented <strong>in</strong> full <strong>in</strong> this submission.<br />
However, a brief overview is provided <strong>in</strong> Appendix 14” (MS, page 38). The <strong>in</strong>clusion criteria clearly<br />
state that phase II-IV trials are <strong>in</strong>cluded and no reference is made to dose-f<strong>in</strong>d<strong>in</strong>g studies be<strong>in</strong>g<br />
excluded. There<strong>for</strong>e it is unclear why this study is treated differently. However, as ADVANCE-1 and<br />
APROPOS used enoxapar<strong>in</strong> 30mg b.d. as <strong>the</strong> comparator, <strong>the</strong> ERG agrees that <strong>the</strong>se trials are not<br />
<strong>in</strong>cluded <strong>in</strong> <strong>the</strong> ma<strong>in</strong> analyses.<br />
In this section we will summarise all evidence from <strong>the</strong> four apixaban trials relat<strong>in</strong>g to <strong>the</strong> outcomes<br />
<strong>in</strong> <strong>the</strong> scope:<br />
- ADVANCE-2: <strong>Apixaban</strong> vs Enoxapar<strong>in</strong> 40 mg o.d. <strong>in</strong> TKR;<br />
- ADVANCE-3: <strong>Apixaban</strong> vs Enoxapar<strong>in</strong> 40 mg o.d. <strong>in</strong> THR;<br />
- ADVANCE-1 and APROPOS: <strong>Apixaban</strong> vs Enoxapar<strong>in</strong> 30 mg b.d. <strong>in</strong> TKR.<br />
None <strong>of</strong> <strong>the</strong> trials reported results <strong>for</strong> jo<strong>in</strong>t outcomes <strong>in</strong>clud<strong>in</strong>g <strong>in</strong>fections, or health related quality <strong>of</strong><br />
life. Pulmonary embolism was reported by three trials as <strong>the</strong> ma<strong>in</strong> post-DVT complication but results<br />
<strong>for</strong> thrombotic syndrome are not presented <strong>in</strong> any trial.<br />
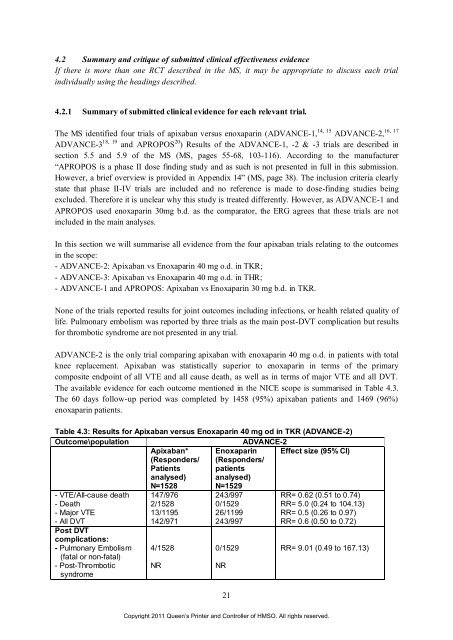
ADVANCE-2 is <strong>the</strong> only trial compar<strong>in</strong>g apixaban with enoxapar<strong>in</strong> 40 mg o.d. <strong>in</strong> patients with total<br />
knee replacement. <strong>Apixaban</strong> was statistically superior to enoxapar<strong>in</strong> <strong>in</strong> terms <strong>of</strong> <strong>the</strong> primary<br />
composite endpo<strong>in</strong>t <strong>of</strong> all VTE and all cause death, as well as <strong>in</strong> terms <strong>of</strong> major VTE and all DVT.<br />
The available evidence <strong>for</strong> each outcome mentioned <strong>in</strong> <strong>the</strong> NICE scope is summarised <strong>in</strong> Table 4.3.<br />
The 60 days follow-up period was completed by 1458 (95%) apixaban patients and 1469 (96%)<br />
enoxapar<strong>in</strong> patients.<br />
Table 4.3: Results <strong>for</strong> <strong>Apixaban</strong> versus Enoxapar<strong>in</strong> 40 mg od <strong>in</strong> TKR (ADVANCE-2)<br />
Outcome\population ADVANCE-2<br />
<strong>Apixaban</strong>* Enoxapar<strong>in</strong> Effect size (95% CI)<br />
(Responders/ (Responders/<br />
Patients patients<br />
analysed) analysed)<br />
N=1528 N=1529<br />
- VTE/All-cause death 147/976 243/997 RR= 0.62 (0.51 to 0.74)<br />
- Death<br />
2/1528<br />
0/1529<br />
RR= 5.0 (0.24 to 104.13)<br />
- Major VTE<br />
13/1195 26/1199 RR= 0.5 (0.26 to 0.97)<br />
- All DVT<br />
Post DVT<br />
complications:<br />
142/971 243/997 RR= 0.6 (0.50 to 0.72)<br />
- Pulmonary Embolism<br />
(fatal or non-fatal)<br />
4/1528<br />
0/1529<br />
RR= 9.01 (0.49 to 167.13)<br />
- Post-Thrombotic<br />
syndrome<br />
NR<br />
NR<br />
21<br />
Copyright 2011 Queen’s Pr<strong>in</strong>ter and Controller <strong>of</strong> HMSO. All rights reserved.