Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5.2 Summary and critique <strong>of</strong> manufacturer’s submitted economic evaluation by <strong>the</strong><br />
ERG<br />
Summarise and critique <strong>the</strong> cost effectiveness evidence submitted by <strong>the</strong> manufacturer<br />
(head<strong>in</strong>gs 5.2.1 to 5.2.11 are suggested head<strong>in</strong>gs). It is noted that <strong>the</strong> ERGs may prefer NOT<br />
to comb<strong>in</strong>e <strong>the</strong> summary and critique <strong>of</strong> <strong>the</strong> submitted economic evidence and <strong>in</strong>stead report<br />
summary and critique sections separately.<br />
Summarise and critique <strong>the</strong> cost effectiveness evidence submitted by <strong>the</strong> manufacturer<br />
(head<strong>in</strong>gs 5.2.1 to 5.2.11 are suggested head<strong>in</strong>gs). It is noted that <strong>the</strong> ERGs may prefer NOT<br />
to comb<strong>in</strong>e <strong>the</strong> summary and critique <strong>of</strong> <strong>the</strong> submitted economic evidence and <strong>in</strong>stead report<br />
summary and critique sections separately.<br />
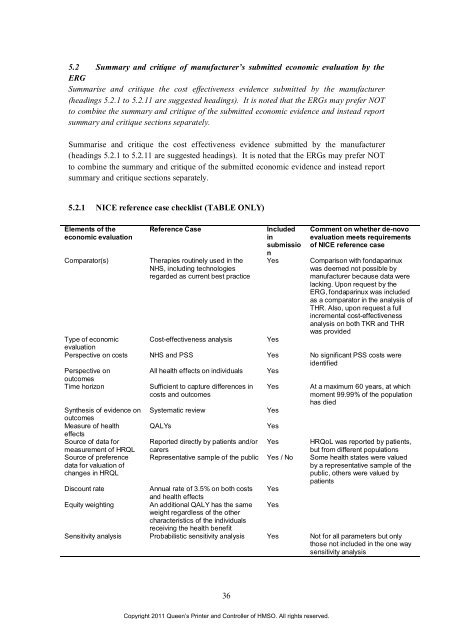
5.2.1 NICE reference case checklist (TABLE ONLY)<br />
Elements <strong>of</strong> <strong>the</strong><br />
economic evaluation<br />
Reference Case Included<br />
<strong>in</strong><br />
submissio<br />
Comparator(s) Therapies rout<strong>in</strong>ely used <strong>in</strong> <strong>the</strong><br />
NHS, <strong>in</strong>clud<strong>in</strong>g technologies<br />
regarded as current best practice<br />
36<br />
n<br />
Comment on whe<strong>the</strong>r de-novo<br />
evaluation meets requirements<br />
<strong>of</strong> NICE reference case<br />
Yes Comparison with fondapar<strong>in</strong>ux<br />
was deemed not possible by<br />
manufacturer because data were<br />
lack<strong>in</strong>g. Upon request by <strong>the</strong><br />
ERG, fondapar<strong>in</strong>ux was <strong>in</strong>cluded<br />
as a comparator <strong>in</strong> <strong>the</strong> analysis <strong>of</strong><br />
THR. Also, upon request a full<br />
<strong>in</strong>cremental cost-effectiveness<br />
analysis on both TKR and THR<br />
was provided<br />
Type <strong>of</strong> economic<br />
evaluation<br />
Cost-effectiveness analysis Yes<br />
Perspective on costs NHS and PSS Yes No significant PSS costs were<br />
identified<br />
Perspective on<br />
outcomes<br />
All health effects on <strong>in</strong>dividuals Yes<br />
Time horizon Sufficient to capture differences <strong>in</strong> Yes At a maximum 60 years, at which<br />
costs and outcomes<br />
moment 99.99% <strong>of</strong> <strong>the</strong> population<br />
has died<br />
Syn<strong>the</strong>sis <strong>of</strong> evidence on<br />
outcomes<br />
Systematic review Yes<br />
Measure <strong>of</strong> health<br />
effects<br />
QALYs Yes<br />
Source <strong>of</strong> data <strong>for</strong> Reported directly by patients and/or Yes HRQoL was reported by patients,<br />
measurement <strong>of</strong> HRQL carers<br />
but from different populations<br />
Source <strong>of</strong> preference Representative sample <strong>of</strong> <strong>the</strong> public Yes / No Some health states were valued<br />
data <strong>for</strong> valuation <strong>of</strong><br />
by a representative sample <strong>of</strong> <strong>the</strong><br />
changes <strong>in</strong> HRQL<br />
public, o<strong>the</strong>rs were valued by<br />
patients<br />
Discount rate Annual rate <strong>of</strong> 3.5% on both costs<br />
and health effects<br />
Yes<br />
Equity weight<strong>in</strong>g An additional QALY has <strong>the</strong> same<br />
weight regardless <strong>of</strong> <strong>the</strong> o<strong>the</strong>r<br />
characteristics <strong>of</strong> <strong>the</strong> <strong>in</strong>dividuals<br />
receiv<strong>in</strong>g <strong>the</strong> health benefit<br />
Yes<br />
Sensitivity analysis Probabilistic sensitivity analysis Yes Not <strong>for</strong> all parameters but only<br />
those not <strong>in</strong>cluded <strong>in</strong> <strong>the</strong> one way<br />
sensitivity analysis<br />
Copyright 2011 Queen’s Pr<strong>in</strong>ter and Controller <strong>of</strong> HMSO. All rights reserved.