Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Comment<br />
– The discount rates and perspective are <strong>in</strong> l<strong>in</strong>e with <strong>the</strong> NICE reference case.<br />
– S<strong>in</strong>ce patients are 65 to 68 years <strong>of</strong> age when <strong>the</strong>y enter <strong>the</strong> model, a 35 year time horizon<br />
seems to reflect lifetime. After 35 years 98% <strong>of</strong> <strong>the</strong> cohort had died.<br />
5.2.6 Treatment effectiveness<br />
Efficacy and safety <strong>of</strong> <strong>the</strong> treatments is modelled <strong>in</strong> l<strong>in</strong>e with <strong>the</strong> correspond<strong>in</strong>g endpo<strong>in</strong>ts <strong>in</strong><br />
<strong>the</strong> ADVANCE, 16, 18 RECORD, 25, 27 RE-MODEL and RE-NOVATE 21, 23 trials:<br />
‘total VTEs and all deaths’ (all adjudicated VTE and all cause death and adjudicated,<br />
symptomatic or asymptomatic DVT, non-fatal PE and death from any cause)<br />
‘total bleeds’ (bleed<strong>in</strong>g at <strong>the</strong> surgical site, non-surgical bleed<strong>in</strong>g events, cl<strong>in</strong>ically<br />
relevant non-major bleed<strong>in</strong>g and m<strong>in</strong>or bleed<strong>in</strong>g events).<br />
It should be noted that this approach to model <strong>the</strong> efficacy and safety <strong>of</strong> <strong>the</strong> comparators does<br />
not allow <strong>for</strong> differences between types <strong>of</strong> bleed (major, m<strong>in</strong>or) and types <strong>of</strong> VTE<br />
(symptomatic, asymptomatic) <strong>for</strong> each comparator <strong>in</strong>dividually.<br />
Enoxapar<strong>in</strong> was <strong>the</strong> reference treatment <strong>in</strong> <strong>the</strong> model. In <strong>the</strong> MS it was stated that both <strong>the</strong><br />
reference treatment rates and <strong>the</strong> apixaban relative risk were taken from <strong>the</strong> ADVANCE-2 16<br />
<strong>for</strong> TKR patients, and from <strong>the</strong> ADVANCE-3 18 <strong>for</strong> THR. In <strong>the</strong> absence <strong>of</strong> head to head RCT<br />
evidence <strong>for</strong> apixaban 2.5 mg bd versus rivaroxaban 10 mg od, and dabigatran 220 mg od, an<br />
adjusted <strong>in</strong>direct comparison approach was adopted. It was stated that because data <strong>for</strong> an<br />
<strong>in</strong>direct comparison with fondapar<strong>in</strong>ux 2.5 mg od was not available, apixaban could not be<br />
compared with fondapar<strong>in</strong>ux <strong>in</strong> <strong>the</strong> model. A primary efficacy population was used <strong>for</strong> ‘total<br />
VTEs and all deaths’, as <strong>the</strong> asymptomatic DVT outcome can only be detected via an<br />
evaluable venogram.<br />
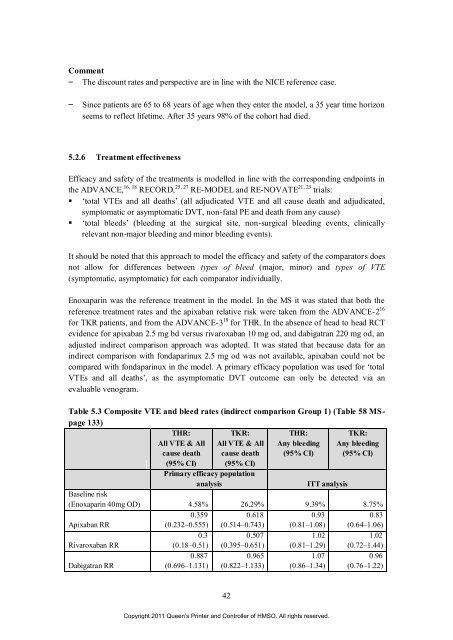
Table 5.3 Composite VTE and bleed rates (<strong>in</strong>direct comparison Group 1) (Table 58 MSpage<br />
133)<br />
THR:<br />
TKR:<br />
THR:<br />
TKR:<br />
All VTE & All All VTE & All Any bleed<strong>in</strong>g Any bleed<strong>in</strong>g<br />
cause death cause death (95% CI) (95% CI)<br />
(95% CI) (95% CI)<br />
Primary efficacy population<br />
analysis ITT analysis<br />
Basel<strong>in</strong>e risk<br />
(Enoxapar<strong>in</strong> 40mg OD) 4.58% 26.29% 9.39% 8.75%<br />
0.359<br />
0.618<br />
0.93<br />
0.83<br />
<strong>Apixaban</strong> RR<br />
(0.232–0.555) (0.514–0.743) (0.81–1.08) (0.64–1.06)<br />
0.3<br />
0.507<br />
1.02<br />
1.02<br />
Rivaroxaban RR<br />
(0.18–0.51) (0.395–0.651) (0.81–1.29) (0.72–1.44)<br />
0.887<br />
0.965<br />
1.07<br />
0.96<br />
Dabigatran RR<br />
(0.696–1.131) (0.822–1.133) (0.86–1.34) (0.76–1.22)<br />
42<br />
Copyright 2011 Queen’s Pr<strong>in</strong>ter and Controller <strong>of</strong> HMSO. All rights reserved.