Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5.2.3 Population<br />
The patient groups <strong>in</strong>cluded <strong>in</strong> <strong>the</strong> economic evaluation are patients aged 18 years and over<br />
who have undergone elective total hip or knee replacement surgery. Patients who have<br />
undergone hip (THR) and knee (TKR) surgery are modelled separately to reflect <strong>the</strong><br />
differences <strong>in</strong> VTE risk, treatment duration, patient characteristics and to reflect <strong>the</strong> appraisal<br />
scope. The populations used <strong>in</strong> <strong>the</strong> model are slightly younger and <strong>for</strong> TKR less <strong>of</strong>ten male<br />
(Table 5.2).<br />
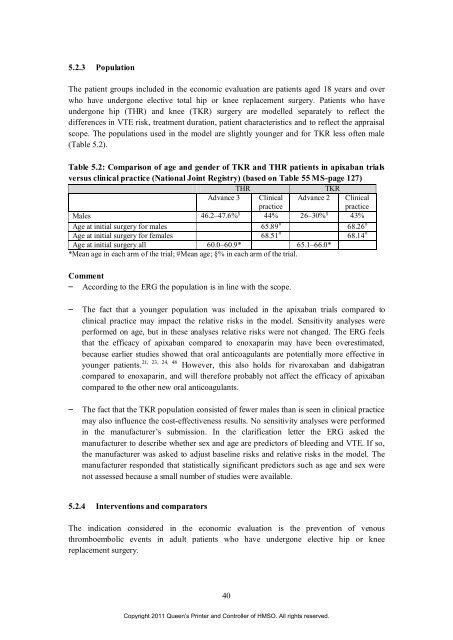
Table 5.2: Comparison <strong>of</strong> age and gender <strong>of</strong> TKR and THR patients <strong>in</strong> apixaban trials<br />
versus cl<strong>in</strong>ical practice (National Jo<strong>in</strong>t Registry) (based on Table 55 MS-page 127)<br />
THR TKR<br />
Advance 3 Cl<strong>in</strong>ical Advance 2 Cl<strong>in</strong>ical<br />
practice<br />
practice<br />
Males 46.2–47.6% § 44% 26–30% § 43%<br />
Age at <strong>in</strong>itial surgery <strong>for</strong> males 65.89 # 68.26 #<br />
Age at <strong>in</strong>itial surgery <strong>for</strong> females 68.51 # 68.14 #<br />
Age at <strong>in</strong>itial surgery all 60.0–60.9* 65.1–66.0*<br />
*Mean age <strong>in</strong> each arm <strong>of</strong> <strong>the</strong> trial; #Mean age; §% <strong>in</strong> each arm <strong>of</strong> <strong>the</strong> trial.<br />
Comment<br />
– Accord<strong>in</strong>g to <strong>the</strong> ERG <strong>the</strong> population is <strong>in</strong> l<strong>in</strong>e with <strong>the</strong> scope.<br />
– The fact that a younger population was <strong>in</strong>cluded <strong>in</strong> <strong>the</strong> apixaban trials compared to<br />
cl<strong>in</strong>ical practice may impact <strong>the</strong> relative risks <strong>in</strong> <strong>the</strong> model. Sensitivity analyses were<br />
per<strong>for</strong>med on age, but <strong>in</strong> <strong>the</strong>se analyses relative risks were not changed. The ERG feels<br />
that <strong>the</strong> efficacy <strong>of</strong> apixaban compared to enoxapar<strong>in</strong> may have been overestimated,<br />
because earlier studies showed that oral anticoagulants are potentially more effective <strong>in</strong><br />
younger patients. 21, 23, 24, 48 However, this also holds <strong>for</strong> rivaroxaban and dabigatran<br />
compared to enoxapar<strong>in</strong>, and will <strong>the</strong>re<strong>for</strong>e probably not affect <strong>the</strong> efficacy <strong>of</strong> apixaban<br />
compared to <strong>the</strong> o<strong>the</strong>r new oral anticoagulants.<br />
– The fact that <strong>the</strong> TKR population consisted <strong>of</strong> fewer males than is seen <strong>in</strong> cl<strong>in</strong>ical practice<br />
may also <strong>in</strong>fluence <strong>the</strong> cost-effectiveness results. No sensitivity analyses were per<strong>for</strong>med<br />
<strong>in</strong> <strong>the</strong> manufacturer’s submission. In <strong>the</strong> clarification letter <strong>the</strong> ERG asked <strong>the</strong><br />
manufacturer to describe whe<strong>the</strong>r sex and age are predictors <strong>of</strong> bleed<strong>in</strong>g and VTE. If so,<br />
<strong>the</strong> manufacturer was asked to adjust basel<strong>in</strong>e risks and relative risks <strong>in</strong> <strong>the</strong> model. The<br />
manufacturer responded that statistically significant predictors such as age and sex were<br />
not assessed because a small number <strong>of</strong> studies were available.<br />
5.2.4 Interventions and comparators<br />
The <strong>in</strong>dication considered <strong>in</strong> <strong>the</strong> economic evaluation is <strong>the</strong> <strong>prevention</strong> <strong>of</strong> <strong>venous</strong><br />
thromboembolic events <strong>in</strong> adult patients who have undergone elective hip or knee<br />
replacement surgery.<br />
40<br />
Copyright 2011 Queen’s Pr<strong>in</strong>ter and Controller <strong>of</strong> HMSO. All rights reserved.