Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Apixaban for the prevention of venous thromboembolism in people ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Post event treatment <strong>in</strong>dependent probabilities<br />
The rema<strong>in</strong><strong>in</strong>g cl<strong>in</strong>ical probabilities <strong>in</strong> <strong>the</strong> decision tree element <strong>of</strong> <strong>the</strong> model were assumed to<br />
be treatment <strong>in</strong>dependent and assumed to not differ between apixaban, enoxapar<strong>in</strong>, dabigatran<br />
and rivaroxaban. As stated by <strong>the</strong> manufacturer, this approach was taken as <strong>the</strong> trials <strong>for</strong><br />
apixaban, rivaroxaban and dabigatran are only powered to detect differences <strong>in</strong> <strong>the</strong> composite<br />
primary efficacy and safety endpo<strong>in</strong>ts. Where possible <strong>the</strong> probabilities <strong>for</strong> <strong>the</strong> post event<br />
treatment <strong>in</strong>dependent probabilities were obta<strong>in</strong>ed from a syn<strong>the</strong>sis <strong>of</strong> all trials on new oral<br />
anticoagulants (all NOAC trials; RECORD RE-MODEL and RE-NOVATE).To syn<strong>the</strong>sise<br />
<strong>the</strong> data, <strong>the</strong> sum <strong>of</strong> events was taken across <strong>the</strong> trials and event types thus provid<strong>in</strong>g a<br />
numerator yield<strong>in</strong>g a total count <strong>for</strong> each event type. The denom<strong>in</strong>ator was obta<strong>in</strong>ed by<br />
summ<strong>in</strong>g all event counts with<strong>in</strong> an endpo<strong>in</strong>t (VTE or any death, Bleeds). If <strong>the</strong> event was not<br />
reported <strong>in</strong> one or more <strong>of</strong> <strong>the</strong> trials, data was extracted from both arms (apixaban and<br />
enoxapar<strong>in</strong>) <strong>of</strong> <strong>the</strong> ADVANCE-2 and 3 trials. As stated, this approach was chosen as <strong>the</strong><br />
number <strong>of</strong> events recorded was small and it was likely that us<strong>in</strong>g apixaban results alone<br />
would <strong>in</strong>troduce chance f<strong>in</strong>d<strong>in</strong>gs and potentially bias <strong>the</strong> results <strong>for</strong> all <strong>the</strong> <strong>in</strong>terventions<br />
evaluated.<br />
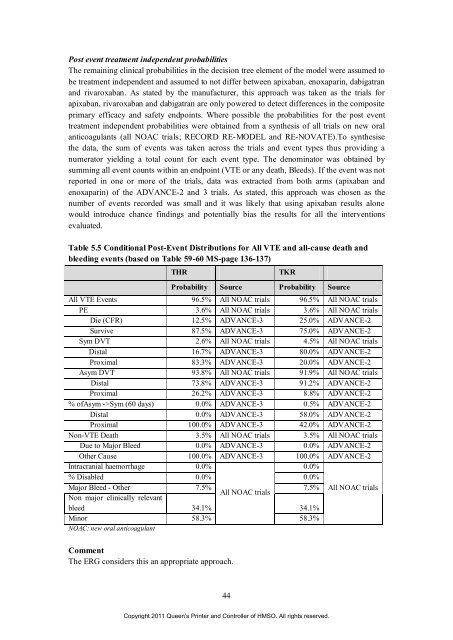
Table 5.5 Conditional Post-Event Distributions <strong>for</strong> All VTE and all-cause death and<br />
bleed<strong>in</strong>g events (based on Table 59-60 MS-page 136-137)<br />
THR TKR<br />
Probability Source Probability Source<br />
All VTE Events 96.5% All NOAC trials 96.5% All NOAC trials<br />
PE 3.6% All NOAC trials 3.6% All NOAC trials<br />
Die (CFR) 12.5% ADVANCE-3 25.0% ADVANCE-2<br />
Survive 87.5% ADVANCE-3 75.0% ADVANCE-2<br />
Sym DVT 2.6% All NOAC trials 4.5% All NOAC trials<br />
Distal 16.7% ADVANCE-3 80.0% ADVANCE-2<br />
Proximal 83.3% ADVANCE-3 20.0% ADVANCE-2<br />
Asym DVT 93.8% All NOAC trials 91.9% All NOAC trials<br />
Distal 73.8% ADVANCE-3 91.2% ADVANCE-2<br />
Proximal 26.2% ADVANCE-3 8.8% ADVANCE-2<br />
% <strong>of</strong>Asym ->Sym (60 days) 0.0% ADVANCE-3 0.5% ADVANCE-2<br />
Distal 0.0% ADVANCE-3 58.0% ADVANCE-2<br />
Proximal 100.0% ADVANCE-3 42.0% ADVANCE-2<br />
Non-VTE Death 3.5% All NOAC trials 3.5% All NOAC trials<br />
Due to Major Bleed 0.0% ADVANCE-3 0.0% ADVANCE-2<br />
O<strong>the</strong>r Cause 100.0% ADVANCE-3 100.0% ADVANCE-2<br />
Intracranial haemorrhage 0.0%<br />
0.0%<br />
% Disabled 0.0% 0.0%<br />
Major Bleed - O<strong>the</strong>r<br />
Non major cl<strong>in</strong>ically relevant<br />
7.5%<br />
All NOAC trials<br />
7.5% All NOAC trials<br />
bleed 34.1% 34.1%<br />
M<strong>in</strong>or<br />
NOAC: new oral anticoagulant<br />
58.3% 58.3%<br />
Comment<br />
The ERG considers this an appropriate approach.<br />
44<br />
Copyright 2011 Queen’s Pr<strong>in</strong>ter and Controller <strong>of</strong> HMSO. All rights reserved.