Rivaroxaban for the treatment of deep vein thrombosis and ...
Rivaroxaban for the treatment of deep vein thrombosis and ...
Rivaroxaban for the treatment of deep vein thrombosis and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
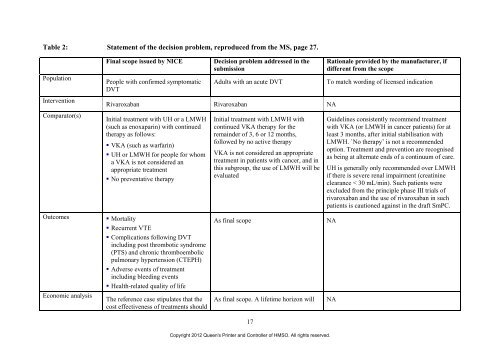
Table 2: Statement <strong>of</strong> <strong>the</strong> decision problem, reproduced from <strong>the</strong> MS, page 27.<br />
Population<br />
Intervention<br />
Comparator(s)<br />
Final scope issued by NICE Decision problem addressed in <strong>the</strong><br />
submission<br />
People with confirmed symptomatic<br />
DVT<br />
17<br />
Rationale provided by <strong>the</strong> manufacturer, if<br />
different from <strong>the</strong> scope<br />
Adults with an acute DVT To match wording <strong>of</strong> licensed indication<br />
<strong>Rivaroxaban</strong> <strong>Rivaroxaban</strong> NA<br />
Initial <strong>treatment</strong> with UH or a LMWH<br />
(such as enoxaparin) with continued<br />
<strong>the</strong>rapy as follows:<br />
VKA (such as warfarin)<br />
UH or LMWH <strong>for</strong> people <strong>for</strong> whom<br />
a VKA is not considered an<br />
appropriate <strong>treatment</strong><br />
No preventative <strong>the</strong>rapy<br />
Outcomes Mortality<br />
Recurrent VTE<br />
Complications following DVT<br />
including post thrombotic syndrome<br />
(PTS) <strong>and</strong> chronic thromboembolic<br />
pulmonary hypertension (CTEPH)<br />
Adverse events <strong>of</strong> <strong>treatment</strong><br />
including bleeding events<br />
Health-related quality <strong>of</strong> life<br />
Economic analysis<br />
The reference case stipulates that <strong>the</strong><br />
cost effectiveness <strong>of</strong> <strong>treatment</strong>s should<br />
Initial <strong>treatment</strong> with LMWH with<br />
continued VKA <strong>the</strong>rapy <strong>for</strong> <strong>the</strong><br />
remainder <strong>of</strong> 3, 6 or 12 months,<br />
followed by no active <strong>the</strong>rapy<br />
VKA is not considered an appropriate<br />
<strong>treatment</strong> in patients with cancer, <strong>and</strong> in<br />
this subgroup, <strong>the</strong> use <strong>of</strong> LMWH will be<br />
evaluated<br />
As final scope NA<br />
As final scope. A lifetime horizon will NA<br />
Copyright 2012 Queen's Printer <strong>and</strong> Controller <strong>of</strong> HMSO. All rights reserved.<br />
Guidelines consistently recommend <strong>treatment</strong><br />
with VKA (or LMWH in cancer patients) <strong>for</strong> at<br />
least 3 months, after initial stabilisation with<br />
LMWH. `No <strong>the</strong>rapy’ is not a recommended<br />
option. Treatment <strong>and</strong> prevention are recognised<br />
as being at alternate ends <strong>of</strong> a continuum <strong>of</strong> care.<br />
UH is generally only recommended over LMWH<br />
if <strong>the</strong>re is severe renal impairment (creatinine<br />
clearance < 30 mL/min). Such patients were<br />
excluded from <strong>the</strong> principle phase III trials <strong>of</strong><br />
rivaroxaban <strong>and</strong> <strong>the</strong> use <strong>of</strong> rivaroxaban in such<br />
patients is cautioned against in <strong>the</strong> draft SmPC.