Rivaroxaban for the treatment of deep vein thrombosis and ...
Rivaroxaban for the treatment of deep vein thrombosis and ...
Rivaroxaban for the treatment of deep vein thrombosis and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
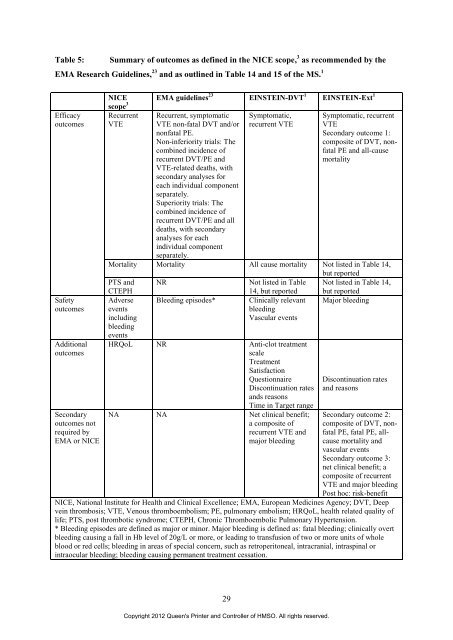
Table 5: Summary <strong>of</strong> outcomes as defined in <strong>the</strong> NICE scope, 3 as recommended by <strong>the</strong><br />
EMA Research Guidelines, 23 <strong>and</strong> as outlined in Table 14 <strong>and</strong> 15 <strong>of</strong> <strong>the</strong> MS. 1<br />
Efficacy<br />
outcomes<br />
NICE<br />
scope 3<br />
Recurrent<br />
VTE<br />
EMA guidelines 23 EINSTEIN-DVT 1 EINSTEIN-Ext 1<br />
Recurrent, symptomatic<br />
VTE non-fatal DVT <strong>and</strong>/or<br />
nonfatal PE.<br />
Non-inferiority trials: The<br />
combined incidence <strong>of</strong><br />
recurrent DVT/PE <strong>and</strong><br />
VTE-related deaths, with<br />
secondary analyses <strong>for</strong><br />
each individual component<br />
separately.<br />
Superiority trials: The<br />
combined incidence <strong>of</strong><br />
recurrent DVT/PE <strong>and</strong> all<br />
deaths, with secondary<br />
analyses <strong>for</strong> each<br />
individual component<br />
separately.<br />
29<br />
Symptomatic,<br />
recurrent VTE<br />
Symptomatic, recurrent<br />
VTE<br />
Secondary outcome 1:<br />
composite <strong>of</strong> DVT, nonfatal<br />
PE <strong>and</strong> all-cause<br />
mortality<br />
Mortality Mortality All cause mortality Not listed in Table 14,<br />
but reported<br />
PTS <strong>and</strong> NR Not listed in Table Not listed in Table 14,<br />
CTEPH<br />
14, but reported but reported<br />
Safety Adverse Bleeding episodes* Clinically relevant Major bleeding<br />
outcomes events<br />
bleeding<br />
including<br />
bleeding<br />
events<br />
Vascular events<br />
Additional HRQoL NR Anti-clot <strong>treatment</strong><br />
outcomes<br />
scale<br />
Treatment<br />
Satisfaction<br />
Questionnaire Discontinuation rates<br />
Discontinuation rates<br />
<strong>and</strong>s reasons<br />
Time in Target range<br />
<strong>and</strong> reasons<br />
Secondary NA NA Net clinical benefit; Secondary outcome 2:<br />
outcomes not<br />
a composite <strong>of</strong> composite <strong>of</strong> DVT, non-<br />
required by<br />
recurrent VTE <strong>and</strong> fatal PE, fatal PE, all-<br />
EMA or NICE<br />
major bleeding cause mortality <strong>and</strong><br />
vascular events<br />
Secondary outcome 3:<br />
net clinical benefit; a<br />
composite <strong>of</strong> recurrent<br />
VTE <strong>and</strong> major bleeding<br />
Post hoc: risk-benefit<br />
NICE, National Institute <strong>for</strong> Health <strong>and</strong> Clinical Excellence; EMA, European Medicines Agency; DVT, Deep<br />
<strong>vein</strong> <strong>thrombosis</strong>; VTE, Venous thromboembolism; PE, pulmonary embolism; HRQoL, health related quality <strong>of</strong><br />
life; PTS, post thrombotic syndrome; CTEPH, Chronic Thromboembolic Pulmonary Hypertension.<br />
* Bleeding episodes are defined as major or minor. Major bleeding is defined as: fatal bleeding; clinically overt<br />
bleeding causing a fall in Hb level <strong>of</strong> 20g/L or more, or leading to transfusion <strong>of</strong> two or more units <strong>of</strong> whole<br />
blood or red cells; bleeding in areas <strong>of</strong> special concern, such as retroperitoneal, intracranial, intraspinal or<br />
intraocular bleeding; bleeding causing permanent <strong>treatment</strong> cessation.<br />
Copyright 2012 Queen's Printer <strong>and</strong> Controller <strong>of</strong> HMSO. All rights reserved.