- Page 1 and 2:
Formaldehyde Tank Cars. Courtesy B.
- Page 3 and 4:
Copyright, 1944, by REIXHOLD PUBLIS
- Page 5 and 6:
iv FORMALDEHYDE of the highest valu
- Page 7 and 8:
Introduction Formaldehyde chemistry
- Page 9 and 10:
Page o y GENERAL IxrRODrcTiON iii P
- Page 11 and 12:
Page CHAPTER IS. HEXAIIETHYLEXEIETR
- Page 13 and 14:
Early History of Formaldehyde FORMA
- Page 15 and 16:
FORMALDEHYDE Production of Formalde
- Page 17 and 18:
6 POEM ALDEHYDE 30-cm hard-glass tu
- Page 19 and 20:
_ 3, "Formaldehyde unit developed b
- Page 21 and 22:
10 FORMALDEHYDE hyde. A modern plan
- Page 23 and 24:
12 FORMALDEHYDE catalysts contain f
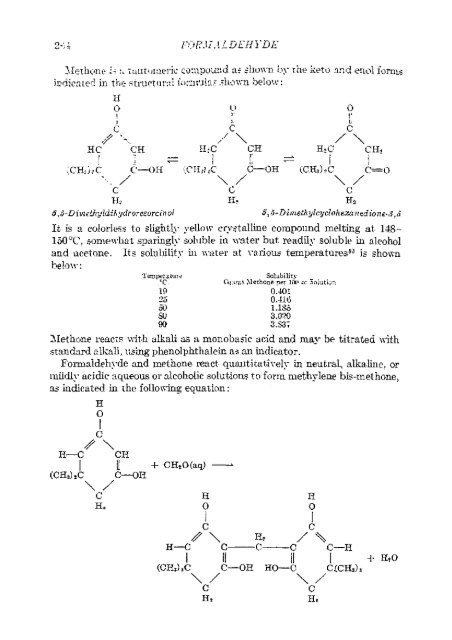
- Page 25 and 26:
14 FORMALDEHYDE decompose at temper
- Page 27 and 28:
18 FORMALDEHYDE comprising aluminum
- Page 29 and 30:
Chapter 2 Monomeric Formaldehyde Al
- Page 31 and 32:
20 FORMALDEHYDE u-av contain small
- Page 33 and 34:
22 FORMALDEHYDE turea from 25 to 12
- Page 35 and 36:
24 FORMALDEHYDE formaldehyde in ace
- Page 37 and 38:
2G FORMALDEHYDE polymerizes very sl
- Page 39 and 40:
Chapter 3 State of Dissolved Formal
- Page 41 and 42:
30 ' FORMALDEHYDE ethylene oxide wi
- Page 43 and 44:
32 FORMALDEHYDE cal equilibrium bet
- Page 45 and 46:
34 FORMALDEHYDE Skrabal and Leutner
- Page 47 and 48:
36 FORMALDEHYDE Equilibria of the f
- Page 49 and 50:
38 FORMALDEHYDE molecule is saturat
- Page 51 and 52:
40 FORMALDEHYDE develop measurable
- Page 53 and 54:
42 ' FORMALDEHYDE present in the mo
- Page 55 and 56:
44 FORMALDEHYDE formaldehyde (methy
- Page 57 and 58:
46 FORMALDEHYDE Toxicity: Physiolog
- Page 59 and 60:
Chapter 5 Physical Properties of Pu
- Page 61 and 62:
50 :H:O/J00 £. Solution 2.23 4.60
- Page 63 and 64:
50 FORMA UihlHYhi: by Natta. and Ba
- Page 65 and 66:
.32 FORMALDEHYDE Partial Pressure,
- Page 67 and 68:
54 FORMALDEHYDE Lacy 17 * has recen
- Page 69 and 70:
50 FORMALDEHYDE At temperatures bel
- Page 71 and 72:
Chapter 6 Distillation of Formaldeh
- Page 73 and 74:
60 FORMALDEHYDE and that the soluti
- Page 75 and 76:
62 FORMALDEHYDE was found to contai
- Page 77 and 78:
Chapter 7 Formaldehyde Polymers Pol
- Page 79 and 80:
60 FORMALDEHYDE true polyoxymethyle
- Page 81 and 82:
6S FORMALDEHYDE sodium sulfate, and
- Page 83 and 84:
70 FORMALDEHYDE formaldehyde soluti
- Page 85 and 86:
72 FORMALDEHYDE ance of a colorless
- Page 87 and 88:
74 FORMALDEHYDE centrationa the rea
- Page 89 and 90:
76 FORMALDEHYDE the vacuum concentr
- Page 91 and 92:
78 FORMALDEHYDE mately 0.1 to 0.3 p
- Page 93 and 94:
80 FORMALDEHYDE dehyde solution was
- Page 95 and 96:
S2 FORMALDEHYDE ingers studies of t
- Page 97 and 98:
Table .17, Proportion at "VidyaxymQ
- Page 99 and 100:
SB FORMALDEHYDE soluble solid diace
- Page 101 and 102:
ss FORMALDEHYDE gradually increases
- Page 103 and 104:
go FORMALDEHYDE sulfuric acid were
- Page 105 and 106:
92 FORMALDEHYDE molecules. This con
- Page 107 and 108:
94 FORMALDEHYDE temperature. Since
- Page 109 and 110:
96 FORMALDEHYDE are forming or evap
- Page 111 and 112:
9S FORMALDEHYDE of formaldehyde to
- Page 113 and 114:
100 FORMALDEHYDE sublimate in the f
- Page 115 and 116:
Chapter 8 ; Chemical Properties of
- Page 117 and 118:
104 FORMALDEHYDE modif}- the decomp
- Page 119 and 120:
106 FORMALDEHYDE Reactions of Forma
- Page 121 and 122:
IMS FORMALDEHYDE i-.n^ ;t!i a-i^-at
- Page 123 and 124:
Ha FORI! ALDEHYDE arid 'ii:„-":.;
- Page 125 and 126:
FORMALDEHYDE \V;:h a-motiii;, :orn^
- Page 127 and 128:
m FORMALDEHYDE Under anhydrous cond
- Page 129 and 130:
116 FORMALDEHYDE 34,1. G. Farbenind
- Page 131 and 132:
118 FORMALDEHYDE The effect of alka
- Page 133 and 134:
120 FORMALDEHYDE precipitates. Coll
- Page 135 and 136:
122 FORMALDEHYDE On heating solutio
- Page 137 and 138:
124 FORMALDEHYDE prepared it by gra
- Page 139 and 140:
126 FORMALDEHYDE By reaction of 960
- Page 141 and 142:
X2S FORMALDEHYDE On reaction of two
- Page 143 and 144:
130 FORMALDEHYDE methylene glycol d
- Page 145 and 146:
132 FORMALDEHYDE Although this acid
- Page 147 and 148:
134 FORMALDEHYDE evolved and carbon
- Page 149 and 150:
m FORMALDEHYDE References I. Anders
- Page 151 and 152:
Chapter 10 Reactions of Formaldehyd
- Page 153 and 154:
ttJSAUTiuivs WITH HYDROXY COMPOUNDS
- Page 155 and 156:
142 FORMALDEHYDE alcohol with forma
- Page 157 and 158:
144 FORMALDEHYDE that no stable con
- Page 159 and 160:
146 FORMALDEHYDE hydrogen halide, a
- Page 161 and 162:
148 FORMALDEHYDE Z, Backer, H. :..
- Page 163 and 164:
Chapter 11 Reactions of Formaldehyd
- Page 165 and 166:
152 FORMALBEH YDE Pentaerythritol i
- Page 167 and 168:
] 54 FORMA LDEHYDB Pentaglyeerol ap
- Page 169 and 170:
lot; CH2OH),C-CHOH,Cf;CEtOH)8 FORMA
- Page 171 and 172:
15S FORM A L DEH YDE In the ease ox
- Page 173 and 174:
1,30 FORMALDEHYDE with cold concent
- Page 175 and 176:
Chapter 12 Reactions of Formaldehyd
- Page 177 and 178:
104 FORMALDEHYDE indicated. rhi> re
- Page 179 and 180:
166 FORMALDEHYDE substituted phenol
- Page 181 and 182:
H3S FORMALDEHYDE The preparation an
- Page 183 and 184:
170 FORMALDEHYDE are slow to react
- Page 185 and 186:
172 FORMALDEHYDE produce methylene
- Page 187 and 188:
174 FORMALDEHYDE The ether, dimethy
- Page 189 and 190:
i7G FORMALDEHYDE ratios, as would b
- Page 191 and 192:
ITS FORMALDEHYDE ami it- polviiueU-
- Page 193 and 194:
ISO FORMALDEHYDE HO^ /> - CH-OCaq)
- Page 195 and 196:
1S2 FORMALDEHYDE On oxidation and h
- Page 197 and 198:
154 FORMALDEHYDE ghieinol itseli ar
- Page 199 and 200:
l&e H 0 T FORMALDEHYDE O il a c / ^
- Page 201 and 202:
1SS FORMALDEHYDE Phenols having thr
- Page 203 and 204:
190 FORMALDEHYDE 27. Eanus, F., J.
- Page 205 and 206:
192 FORMALDEHYDE Acids containing a
- Page 207 and 208:
194 FORMALDEHYDE reaction which tak
- Page 209 and 210:
190 FORMALDEHYDE era! hiji;rs. When
- Page 211 and 212:
19S FORMALDEHYDE By oxidation with
- Page 213 and 214:
200 FORMALDEHYDE cold reaction mixt
- Page 215 and 216:
202 FORMALDEHYDE In the case of eth
- Page 217 and 218:
204 FORMALDEHYDE *m \it.^:U:Z hi mt
- Page 219 and 220:
200 FORMALDEHYDE In av^^iu:, -econd
- Page 221 and 222:
2QS FORMALDEHYDE Mixed methylene de
- Page 223 and 224:
•210 FORMALDEHYDE According to Ka
- Page 225 and 226: 12 FORMALDEHYDE Tho t>*.:y:;.*:'k-
- Page 227 and 228: 214 FORMALDEHYDE hy employing an ac
- Page 229 and 230: 2IM FORMA LDEH YDE In the presence
- Page 231 and 232: 21S FORMALDEHYDE On exposure TO dil
- Page 233 and 234: 220 FORMALDEHYDE •with 4 or more
- Page 235 and 236: 222 FORMALDEHYDE anism by which the
- Page 237 and 238: 224 FORMALDEHYDE H'iris \.\\i. :\j:
- Page 239 and 240: 220 FORMALDEHYDE 97. SeieiEer, E, T
- Page 241 and 242: 22S FORMALDEHYDE Since strongly aci
- Page 243 and 244: 230 FOEMALDBBYDE feolsble derivativ
- Page 245 and 246: 232 HOCHjCi - j V / u H H CH2C! FOR
- Page 247 and 248: 234 CHj CHa / \ FORMALDEHYDE CHs /
- Page 249 and 250: 2m FORMALDEHYDE extremely stringent
- Page 251 and 252: 23S FORMALDEHYDE silver aeervHde an
- Page 253 and 254: 24.0 FORMALDEHYDE alcohol*. Moureu
- Page 255 and 256: 242 FORMALDEHYDE Farbenindusnie pat
- Page 257 and 258: Chapter 16 Detection and Estimation
- Page 259 and 260: 24ti FORMALDEHYDE iiiriue :'-r vrr.
- Page 261 and 262: *>JS FOIOIALBEHYD, t-,T^iK^ed «JJ
- Page 263 and 264: 250 FORMALDEHYDE Beta-Naphthoi. Bet
- Page 265 and 266: 252 FORMALDEHYDE bridle compounds o
- Page 267 and 268: 254 FORMALDEHYDE 14. Dtoigts, G., C
- Page 269 and 270: 250 FORMALDEHYDE Formaldehyde may a
- Page 271 and 272: 23S FORMALDEHYDE analyzed, but shou
- Page 273 and 274: 2u0 FORMALDEHYDE by Roniijn :: . Th
- Page 275: 262 FORMALDEHYDE The following modi
- Page 279 and 280: 26G FORMALDEHYDE should be neutral
- Page 281 and 282: 2i kS FORMALDEHYDE previously m^-ur
- Page 283 and 284: 270 FORMALDEHYDE tioii of ike metha
- Page 285 and 286: 272 FORMALDEHYDE insoluble material
- Page 287 and 288: 274 FORMALDEHYDE hyde liberated by
- Page 289 and 290: Chapter 18 Hexamethylenetetramine H
- Page 291 and 292: 27S FORMALDEHYDE show monobasic cha
- Page 293 and 294: 2S0 FORMALDEH YDE Reactions I and I
- Page 295 and 296: 2S2 FORMALDEHYDE 275"F 135 ; C/. By
- Page 297 and 298: 2S4 FORMALDEHYDE On ignition, it bu
- Page 299 and 300: 2S6 FORMALDEHYDE Physiological Prop
- Page 301 and 302: 2SS FORMA LDEH YDE base or ?JV :he
- Page 303 and 304: 290 FORMALDEHYDE Reaction* with Sul
- Page 305 and 306: 292 FORMA LDEH1 'BE erately concent
- Page 307 and 308: 294 FORMALDEHYDE acetone in boiling
- Page 309 and 310: 296 FORMALDEHYDE for 15 minute* wit
- Page 311 and 312: 298 FORMALDEHYDE approximately 0/2
- Page 313 and 314: 300 FORMALDEHYDE 35. D^v-jkv. -T. V
- Page 315 and 316: Chapter 19 Uses of Formaldehyde, Fo
- Page 317 and 318: 304 ' FORMA LDEH YJj£ of both plan
- Page 319 and 320: 306 FORMALDEHYDE ent, resistant to
- Page 321 and 322: 305 FORMALDEHYDE Resins, joining wo
- Page 323 and 324: 310 FORMALDEHYDE has played an incr
- Page 325 and 326: 312 FORMA LB Ell 3 V D£ resin-like
- Page 327 and 328:
314 FORMALDEHYDE For example, it is
- Page 329 and 330:
316 FORMALDEHYDE ically involved in
- Page 331 and 332:
Chapter 20 Uses of Formaldehyde, Fo
- Page 333 and 334:
320 FORMALDEHYDE 12-24 hours-. Some
- Page 335 and 336:
322 FORMALDEHYDE has been utilized
- Page 337 and 338:
324 FORMALDEHYDE References 1. Baue
- Page 339 and 340:
326 FORMALDEHYDE soaking for four t
- Page 341 and 342:
32S FORMALDEHYDE colors is among th
- Page 343 and 344:
330 FORMALDEHYDE 7. Jones, H. I., !
- Page 345 and 346:
332 FORMALDEHYDE Tetranifcthylenedi
- Page 347 and 348:
334 FORMALDEHYDE 0. Rinser, F., :o
- Page 349 and 350:
33(3 FORMA LDEHYDE low dishes or on
- Page 351 and 352:
338 FORMALDEHYDE sistance and other
- Page 353 and 354:
340 FORMALDEHYDE complUhed by deriv
- Page 355 and 356:
M2 FORMALDEHYDE is reported thai t'
- Page 357 and 358:
344 FORMALDEHYDE References 1. Bore
- Page 359 and 360:
341) FORMALDEHYDE distilled water a
- Page 361 and 362:
34S FORMALDEHYDE high wet-strength
- Page 363 and 364:
350 FORMALDEHYDE a roll, after whic
- Page 365 and 366:
35*2 FORMA LDEH \ 'DK chloride "lit
- Page 367 and 368:
354 FORMALDEHYDE forming developers
- Page 369 and 370:
356 FORMALDEHYDE In addition, it is
- Page 371 and 372:
35S FORMALDEHYDE An entirely differ
- Page 373 and 374:
360 FORMALDEHYDE According to Seymo
- Page 375 and 376:
362 FORMALDEHYDE improved by an aci
- Page 377 and 378:
364 FORMALDEHYDE type because the p
- Page 379 and 380:
366* FORMALDEHYDE spiration can be
- Page 381 and 382:
36S FORMALDEHYDE itatrng bath which
- Page 383 and 384:
370 FORMALDEHYDE 39. Lantz, L. A.,
- Page 385 and 386:
Abel, JM 1S1 Acres, S. F., 125 Adam
- Page 387 and 388:
Lacy, B. S.t 54 Ladisck, C, 215 Lae
- Page 389 and 390:
IB, 1, Itotaf,!,! Ifflt,0,S i,! ife
- Page 391 and 392:
Ammonia, reaction, of formaldehyde
- Page 393 and 394:
Cyclic polymers, 65; 94-100 Tetraox
- Page 395 and 396:
Hydrocarbon gases, production from,
- Page 397 and 398:
Polyeondensation of simple, 112 Rea
- Page 399 and 400:
Lower polyoxymethylene glycols, 67-
- Page 401 and 402:
Aromatic hydrocarbons, 231-237 Dlar
- Page 403 and 404:
Latex, 355-356 Synthetic, 358 Salic
- Page 405 and 406:
,- T7 Dyes, 327-329 Embalming, 329-
- Page 407 and 408:
No. 35. Noxious Gases. By Yandell H