BioProcess Product Guide - GE Healthcare Life Sciences
BioProcess Product Guide - GE Healthcare Life Sciences
BioProcess Product Guide - GE Healthcare Life Sciences
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Real-time control<br />
Up to four systems can be connected to one UNICORN<br />
workstation where individual controllers handle the realtime<br />
control of each system. Data evaluation or methods<br />
programming can be done while the systems are running.<br />
The control unit CU 950 (Ethernet and USB) provides a high<br />
degree of security for control and data. The unit secures<br />
started runs even if the local PC and communication is<br />
disrupted. CU 950 Advanced also contains an internal<br />
memory that collects data in case of communication<br />
failure.<br />
Extensive data evaluation<br />
All monitor data are stored in a Result File for storage and<br />
evaluation. Extensive data processing routines include<br />
curve smoothing, differentiation, normalization, baseline<br />
calculation, peak integration and height equivalent to a<br />
theoretical plate (HETP) calculations.<br />
Start protocols are user-defined questions that must be<br />
answered before a run can be started. Questions can<br />
vary between simple operator prompts to those requiring<br />
mandatory answers and authorized approval.<br />
Notebooks permit additional text to be included in the<br />
process documentation. Separate files are generated for<br />
method notes, start notes, run notes, and evaluation notes.<br />
These notes can be entered at the designated time and<br />
cannot be altered after the run is complete.<br />
All programmed and manual events occurring during the<br />
run, including alarms and warnings, are documented in the<br />
logbook and cannot be altered.<br />
Security<br />
UNICORN provides a system for password authorization<br />
and access control. Operators log in by name and<br />
password. The user profile includes an access level that<br />
defines system functions available to each operator.<br />
Scalability<br />
Using UNICORN for method or process development on<br />
ÄKTA systems simplifies scale-up to <strong>BioProcess</strong> system.<br />
Personnel retraining is minimized and continuity exists in<br />
batch documentation and report generation.<br />
6<br />
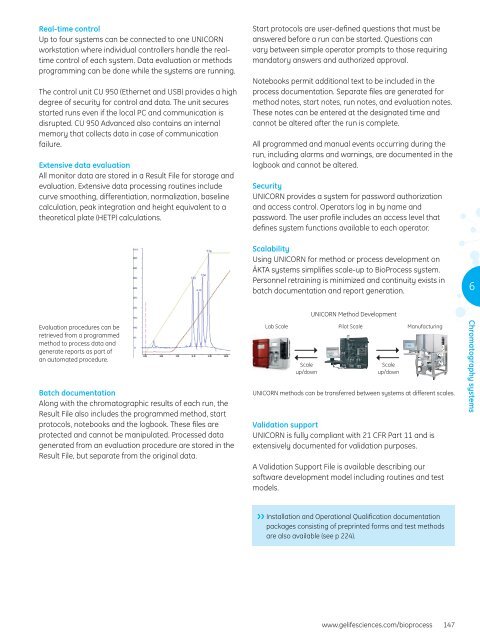
UNICORN Method Development<br />
Evaluation procedures can be<br />
retrieved from a programmed<br />
method to process data and<br />
generate reports as part of<br />
an automated procedure.<br />
Batch documentation<br />
Along with the chromatographic results of each run, the<br />
Result File also includes the programmed method, start<br />
protocols, notebooks and the logbook. These files are<br />
protected and cannot be manipulated. Processed data<br />
generated from an evaluation procedure are stored in the<br />
Result File, but separate from the original data.<br />
Lab Scale Pilot Scale Manufacturing<br />
Scale<br />
up/down<br />
Scale<br />
up/down<br />
UNICORN methods can be transferred between systems at different scales.<br />
Validation support<br />
UNICORN is fully compliant with 21 CFR Part 11 and is<br />
extensively documented for validation purposes.<br />
A Validation Support File is available describing our<br />
software development model including routines and test<br />
models.<br />
Chromatography systems<br />
Installation and Operational Qualification documentation<br />
packages consisting of preprinted forms and test methods<br />
are also available (see p 224).<br />
www.gelifesciences.com/bioprocess 147