Pre-Lab 13 Assignment: Radioactive Decay
Pre-Lab 13 Assignment: Radioactive Decay
Pre-Lab 13 Assignment: Radioactive Decay
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Radioactive</strong> <strong>Decay</strong> v 0.1<br />
sample is proportional to the number of nuclei in the sample<br />
∆N<br />
∆t<br />
= −λN, (3)<br />
the number of nuclei remaining in the sample will follow an exponential law:<br />
N = N 0 e −λt (4)<br />
where λ is the decay constant and has unit of inverse time, and N 0 is the number of nuclei<br />
present in the sample before any decays occur. In this lab we will investigate the decay of<br />
radioactive samples and the relationship between the decay constant and the half-life and mean<br />
life of a radioactive material.<br />
Investigation 1:<br />
Half-Life and Mean Life of an Isotope<br />
Eq. (4) describes the exponential decay in terms of the decay constant λ, however one can<br />
rewrite it in a way very similar to the decay in the RC circuit described by Eq. (2). The mean<br />
life (τ) of a certain unstable nuclei is defined as τ = 1/λ. And Eq. (4) becomes<br />
N = N 0 e −t/τ . (1.5)<br />
Just like the RC circuit τ is the time constant that tells us how fast or how slow the sample will<br />
decay. Its meaning is that one expects, on average, that a particular nuclei will decay within<br />
this characteristic time. But, of course, some nuclei will decay faster and others will decay<br />
slower than τ. τ is determined exactly the same way as we did for the RC circuit, that is we<br />
determine how long it takes the sample to have 37 % of the original number of nuclei. Another<br />
quantity of interest is the half-life of the isotope, which is defined as the time it takes for the<br />
sample to have 50 % of the original number of parent nuclei N 0 to decay. By solving Eq. (4)<br />
for T 1/2 we obtain<br />
T 1/2 = ln2<br />
λ = 0.693<br />
λ . (1.6)<br />
Question 1.1 Solve Eq. (4) with the condition that at t = T 1/2 , N = N 0 /2 and prove Eq. (1.6).<br />
Show all your work<br />
One of the common uses of radioactive decays is carbon dating. Carbon dating is done by<br />
measuring the rate of decay (or ”activity”, the number of decays per unit time) of the 14 6C<br />
isotope in a sample of an artifact made of organic material. It can be shown that the activity<br />
follows the same exponential law as the number of parent nuclei in the sample, i.e.<br />
(<br />
∆N ∆N<br />
∆t = ∆t<br />
)<br />
e −λt , (1.7)<br />
0<br />
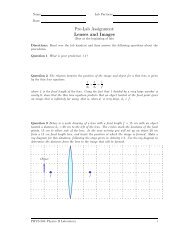
where (∆N/∆t) 0 is the initial activity of the sample. In the axes below we show the activity<br />
of a sample of 14 6C as a function of time.<br />
PHYS-204:Physics II <strong>Lab</strong>oratory 3