Nuclear Spectroscopy
Nuclear Spectroscopy
Nuclear Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
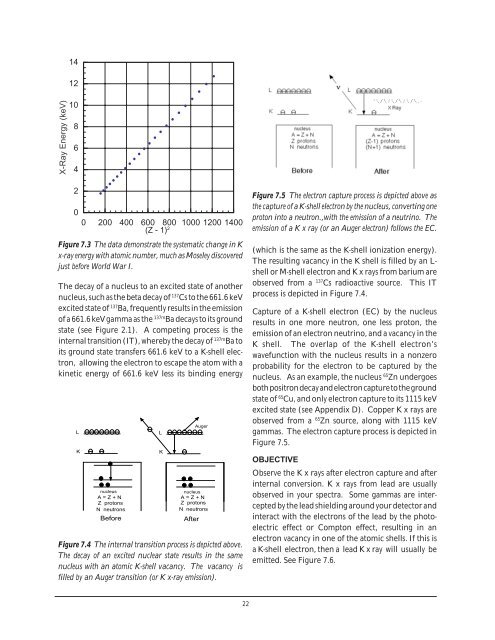
14<br />
12<br />
X-Ray Energy (keV)<br />
10<br />
8<br />
6<br />
4<br />
2<br />
X XXXXXXXXXXXXXXXXXXXXXX<br />
0<br />
0 200 400 600 800 1000 1200 1400<br />
(Z - 1) 2<br />
Figure 7.3 The data demonstrate the systematic change in K<br />
x-ray energy with atomic number, much as Moseley discovered<br />
just before World War I.<br />
The decay of a nucleus to an excited state of another<br />
nucleus, such as the beta decay of 137 Cs to the 661.6 keV<br />
excited state of 137 Ba, frequently results in the emission<br />
of a 661.6 keV gamma as the 137m Ba decays to its ground<br />
state (see Figure 2.1). A competing process is the<br />
internal transition (IT), whereby the decay of 137m Ba to<br />
its ground state transfers 661.6 keV to a K-shell electron,<br />
allowing the electron to escape the atom with a<br />
kinetic energy of 661.6 keV less its binding energy<br />
L<br />
K<br />
nucleus<br />
A = Z + N<br />
Z protons<br />
N neutrons<br />
Before<br />
Auger<br />
Figure 7.4 The internal transition process is depicted above.<br />
The decay of an excited nuclear state results in the same<br />
nucleus with an atomic K-shell vacancy. The vacancy is<br />
filled by an Auger transition (or K x-ray emission).<br />
L<br />
K<br />
nucleus<br />
A = Z + N<br />
Z protons<br />
N neutrons<br />
After<br />
Figure 7.5 The electron capture process is depicted above as<br />
the capture of a K-shell electron by the nucleus, converting one<br />
proton into a neutron.,with the emission of a neutrino. The<br />
emission of a K x ray (or an Auger electron) follows the EC.<br />
(which is the same as the K-shell ionization energy).<br />
The resulting vacancy in the K shell is filled by an L-<br />
shell or M-shell electron and K x rays from barium are<br />
observed from a 137 Cs radioactive source. This IT<br />
process is depicted in Figure 7.4.<br />
Capture of a K-shell electron (EC) by the nucleus<br />
results in one more neutron, one less proton, the<br />
emission of an electron neutrino, and a vacancy in the<br />
K shell. The overlap of the K-shell electron’s<br />
wavefunction with the nucleus results in a nonzero<br />
probability for the electron to be captured by the<br />
nucleus. As an example, the nucleus 65 Zn undergoes<br />
both positron decay and electron capture to the ground<br />
state of 65 Cu, and only electron capture to its 1115 keV<br />
excited state (see Appendix D). Copper K x rays are<br />
observed from a 65 Zn source, along with 1115 keV<br />
gammas. The electron capture process is depicted in<br />
Figure 7.5.<br />
OBJECTIVE<br />
Observe the K x rays after electron capture and after<br />
internal conversion. K x rays from lead are usually<br />
observed in your spectra. Some gammas are intercepted<br />
by the lead shielding around your detector and<br />
interact with the electrons of the lead by the photoelectric<br />
effect or Compton effect, resulting in an<br />
electron vacancy in one of the atomic shells. If this is<br />
a K-shell electron, then a lead K x ray will usually be<br />
emitted. See Figure 7.6.<br />
22