Nuclear Spectroscopy
Nuclear Spectroscopy
Nuclear Spectroscopy
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
E X P E R I M E N T 13<br />
Uranium 238 Series<br />
INTRODUCTION<br />
Like thorium, uranium is found is most building<br />
materials and soils on the earth. There are three<br />
isotopes of uranium still found abundantly in the<br />
earth’s crust. Mass 238 is the most abundant (99.28%),<br />
and the other isotopes are much less abundant, 235 U<br />
(0.72%), 234 U (0.0055%).<br />
The vast difference in the relative abundances of 238 U<br />
and 235 U is supposedly due to the factor of 6 difference<br />
in their half-lives (see Tables 13.1 and 13.2), and<br />
the long time from the creation of nearly equal<br />
quantities of the two isotopes. This was about 6 x 10 9<br />
years ago, the postulated time for the supernova<br />
whose remnants eventually became our solar system.<br />
The presence of 234 U after this great length of time is<br />
due only to its being created in the decay chain<br />
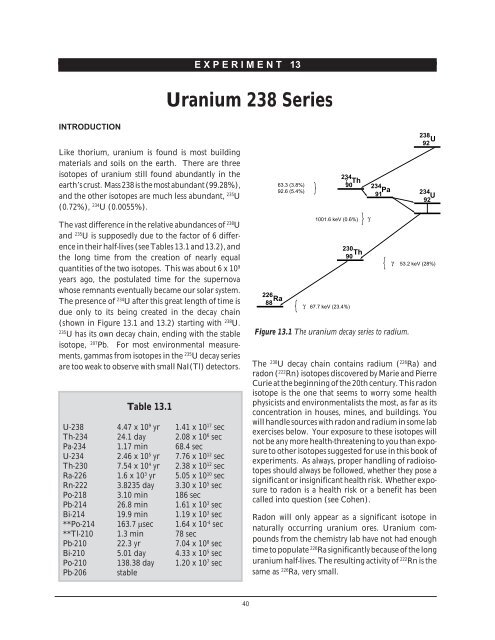
(shown in Figure 13.1 and 13.2) starting with 238 U.<br />
235<br />
U has its own decay chain, ending with the stable<br />
isotope, 207 Pb. For most environmental measurements,<br />
gammas from isotopes in the 235 U decay series<br />
are too weak to observe with small NaI(Tl) detectors.<br />
Table 13.1<br />
U-238 4.47 x 10 9 yr 1.41 x 10 17 sec<br />
Th-234 24.1 day 2.08 x 10 6 sec<br />
Pa-234 1.17 min 68.4 sec<br />
U-234 2.46 x 10 5 yr 7.76 x 10 12 sec<br />
Th-230 7.54 x 10 4 yr 2.38 x 10 12 sec<br />
Ra-226 1.6 x 10 3 yr 5.05 x 10 10 sec<br />
Rn-222 3.8235 day 3.30 x 10 5 sec<br />
Po-218 3.10 min 186 sec<br />
Pb-214 26.8 min 1.61 x 10 3 sec<br />
Bi-214 19.9 min 1.19 x 10 3 sec<br />
**Po-214 163.7 µsec 1.64 x 10 -4 sec<br />
**Tl-210 1.3 min 78 sec<br />
Pb-210 22.3 yr 7.04 x 10 8 sec<br />
Bi-210 5.01 day 4.33 x 10 5 sec<br />
Po-210 138.38 day 1.20 x 10 7 sec<br />
Pb-206 stable<br />
226<br />
Ra<br />
88<br />
63.3 (3.8%)<br />
92.6 (5.4%)<br />
234 γ Th<br />
90<br />
1001.6 keV (0.6%)<br />
γ 67.7 keV (23.4%)<br />
230<br />
Th<br />
90<br />
234<br />
Pa<br />
91<br />
Figure 13.1 The uranium decay series to radium.<br />
238<br />
U<br />
92<br />
The 238 U decay chain contains radium ( 226 Ra) and<br />
radon ( 222 Rn) isotopes discovered by Marie and Pierre<br />
Curie at the beginning of the 20th century. This radon<br />
isotope is the one that seems to worry some health<br />
physicists and environmentalists the most, as far as its<br />
concentration in houses, mines, and buildings. You<br />
will handle sources with radon and radium in some lab<br />
exercises below. Your exposure to these isotopes will<br />
not be any more health-threatening to you than exposure<br />
to other isotopes suggested for use in this book of<br />
experiments. As always, proper handling of radioisotopes<br />
should always be followed, whether they pose a<br />
significant or insignificant health risk. Whether exposure<br />
to radon is a health risk or a benefit has been<br />
called into question (see Cohen).<br />
Radon will only appear as a significant isotope in<br />
naturally occurring uranium ores. Uranium compounds<br />
from the chemistry lab have not had enough<br />
time to populate 226 Ra significantly because of the long<br />
uranium half-lives. The resulting activity of 222 Rn is the<br />
same as 226 Ra, very small.<br />
γ<br />
U<br />
234<br />
92<br />
γ 53.2 keV (28%)<br />
40