Nuclear Spectroscopy
Nuclear Spectroscopy
Nuclear Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
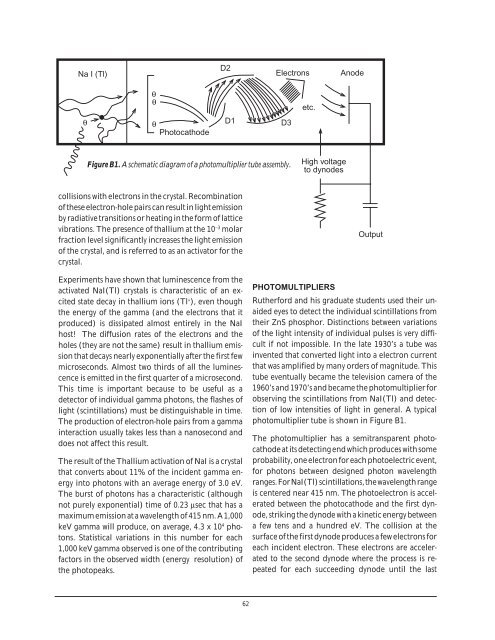
Na I (Tl)<br />
D2<br />
Electrons<br />
Anode<br />
θ<br />
θ<br />
θ<br />
etc.<br />
θ<br />
Photocathode<br />
D1<br />
D3<br />
Figure B1. A schematic diagram of a photomultiplier tube assembly.<br />
High voltage<br />
to dynodes<br />
collisions with electrons in the crystal. Recombination<br />
of these electron-hole pairs can result in light emission<br />
by radiative transitions or heating in the form of lattice<br />
vibrations. The presence of thallium at the 10 −3 molar<br />
fraction level significantly increases the light emission<br />
of the crystal, and is referred to as an activator for the<br />
crystal.<br />
Experiments have shown that luminescence from the<br />
activated NaI(Tl) crystals is characteristic of an excited<br />
state decay in thallium ions (Tl + ), even though<br />
the energy of the gamma (and the electrons that it<br />
produced) is dissipated almost entirely in the NaI<br />
host! The diffusion rates of the electrons and the<br />
holes (they are not the same) result in thallium emission<br />
that decays nearly exponentially after the first few<br />
microseconds. Almost two thirds of all the luminescence<br />
is emitted in the first quarter of a microsecond.<br />
This time is important because to be useful as a<br />
detector of individual gamma photons, the flashes of<br />
light (scintillations) must be distinguishable in time.<br />
The production of electron-hole pairs from a gamma<br />
interaction usually takes less than a nanosecond and<br />
does not affect this result.<br />
The result of the Thallium activation of NaI is a crystal<br />
that converts about 11% of the incident gamma energy<br />
into photons with an average energy of 3.0 eV.<br />
The burst of photons has a characteristic (although<br />
not purely exponential) time of 0.23 µsec that has a<br />
maximum emission at a wavelength of 415 nm. A 1,000<br />
keV gamma will produce, on average, 4.3 x 10 4 photons.<br />
Statistical variations in this number for each<br />
1,000 keV gamma observed is one of the contributing<br />
factors in the observed width (energy resolution) of<br />
the photopeaks.<br />
Output<br />
PHOTOMULTIPLIERS<br />
Rutherford and his graduate students used their unaided<br />
eyes to detect the individual scintillations from<br />
their ZnS phosphor. Distinctions between variations<br />
of the light intensity of individual pulses is very difficult<br />
if not impossible. In the late 1930’s a tube was<br />
invented that converted light into a electron current<br />
that was amplified by many orders of magnitude. This<br />
tube eventually became the television camera of the<br />
1960’s and 1970’s and became the photomultiplier for<br />
observing the scintillations from NaI(Tl) and detection<br />
of low intensities of light in general. A typical<br />
photomultiplier tube is shown in Figure B1.<br />
The photomultiplier has a semitransparent photocathode<br />
at its detecting end which produces with some<br />
probability, one electron for each photoelectric event,<br />
for photons between designed photon wavelength<br />
ranges. For NaI(Tl) scintillations, the wavelength range<br />
is centered near 415 nm. The photoelectron is accelerated<br />
between the photocathode and the first dynode,<br />
striking the dynode with a kinetic energy between<br />
a few tens and a hundred eV. The collision at the<br />
surface of the first dynode produces a few electrons for<br />
each incident electron. These electrons are accelerated<br />
to the second dynode where the process is repeated<br />
for each succeeding dynode until the last<br />
62