download (pdf, 6MB) - SNV
download (pdf, 6MB) - SNV
download (pdf, 6MB) - SNV
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
catabolize acetate and H 2 to gaseous products in the absence of exogenous electron accepiors other than CO 2<br />
or light energy. In their absence effective degradation would cease because of accumulation of non gaseous<br />
reduced fatty acid and alcohol products of the fermentative and other H 2 using bacteria that have almost the<br />
same energy content as the original organic matter.<br />
In morphology they are of different types of such as cocci, bacilli, spiralli and sarcinea. In 1940, Barker<br />
isolated a pure culture of Methanobacterium omehanskii capable of reducing carbon dioxide into methane. In<br />
1956, based upon morphology, Barker classified the methane producing bacteria into the following four<br />
groups. Carbon substrates are oxidized by methogenic bacteria to form methane as given in Table 3.1.<br />
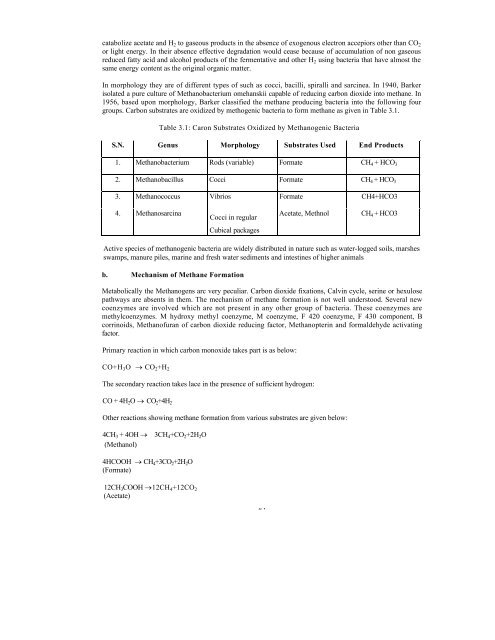
Table 3.1: Caron Substrates Oxidized by Methanogenic Bacteria<br />
S.N. Genus Morphology Substrates Used End Products<br />
1. Methanobacterium Rods (variable) Formate CH 4 + HCO 3<br />
2. Methanobacillus Cocci Formate CH 4 + HCO 3<br />
3. Methanococcus Vibrios Formate CH4+HCO3<br />
4. Methanosarcina<br />
Cocci in regular<br />
Acetate, Methnol<br />
CH 4 + HCO3<br />
Cubical packages<br />
Active species of methanogenic bacteria are widely distributed in nature such as water-logged soils, marshes<br />
swamps, manure piles, marine and fresh water sediments and intestines of higher animals<br />
b. Mechanism of Methane Formation<br />
Metabolically the Methanogens arc very peculiar. Carbon dioxide fixations, Calvin cycle, serine or hexulose<br />
pathways are absents in them. The mechanism of methane formation is not well understood. Several new<br />
coenzymes are involved which are not present in any other group of bacteria. These coenzymes are<br />
methylcoenzymes. M hydroxy methyl coenzyme, M coenzyme, F 420 coenzyme, F 430 component, B<br />
corrinoids, Methanofuran of carbon dioxide reducing factor, Methanopterin and formaldehyde activating<br />
factor.<br />
Primary reaction in which carbon monoxide takes part is as below:<br />
CO+H 3 O → CO 2 +H 2<br />
The secondary reaction takes lace in the presence of sufficient hydrogen:<br />
CO + 4H 2 O → CO 2 +4H 2<br />
Other reactions showing methane formation from various substrates are given below:<br />
4CH 3 + 4OH → 3CH 4 +CO 2 +2H 2 O<br />
(Methanol)<br />
4HCOOH → CH 4 +3CO 2 +2H 2 O<br />
(Formate)<br />
12CH 3 COOH →12CH 4 +12CO 2<br />
(Acetate)<br />
24