Ground Truth Studies Teacher Handbook - Aspen Global Change ...
Ground Truth Studies Teacher Handbook - Aspen Global Change ...
Ground Truth Studies Teacher Handbook - Aspen Global Change ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
A <strong>Global</strong> <strong>Change</strong> Primer (continued)<br />
Methane<br />
Methane, the second most significant greenhouse gas has as primary natural sources wetlands and termite metabolism.<br />
Anthropogenic sources are coal mining, leaks in natural gas systems, rice paddies, enteric fermentation from the digestion<br />
of grazing animals like cows, from animal wastes, landfills and biomass burning. Methane concentration in the atmosphere<br />
has more than doubled from its pre-industrial levels and until recently was increasing at about 1% per year. Over<br />
the last decade this rate has slowed and is now at about 0.6%. This is still a significant rate, especially when one<br />
considers that each molecule of methane has many times the warming potential as a molecule of carbon dioxide (see<br />
<strong>Global</strong> Warming Potential (GWP) below).<br />
CFCs<br />
Not all greenhouse gases are naturally occurring like carbon dioxide or methane. Some of the trace gases are artificially<br />
manufactured and are new to this century. They include the chloroflurocarbons (CFCs), invented in the 1930s, and the<br />
recently developed CFC-replacement family of hydrogenated chloroflurocarbons (HCFCs), hydrogenated fluorocarbons<br />
(HFCs), and various compounds with bromine (halons). These trace gases often have long lifetimes in the atmosphere,<br />
and on a per molecule basis, some are equivalent to thousands of CO 2<br />
molecules in their greenhouse heat-rapping effect.<br />
Coincidentally, CFCs and related chemicals are also responsible for the loss of ozone in the stratosphere due to the<br />
release of chlorine and bromine when the compounds are transported by convection to the stratosphere and are exposed to<br />
more intense ultraviolet light. Chlorine and bromine catalyze the destruction of ozone above natural rates. More about<br />
CFCs and ozone later. Recent research has indicated that ozone depleting chemicals such as the CFCs, that have a direct<br />
warming effect as greenhouse gases, also have an indirect cooling effect by removing ozone, which itself is a greenhouse<br />
gas from the lower stratosphere. Because of this development, the net effect of some of the halocarbons as greenhouse<br />
gases is unclear.<br />
Tropospheric ozone<br />
Tropospheric Ozone is a greenhouse gas of<br />
increasing significance. This lower atmosphere<br />
ozone is photochemically (chemical reaction<br />
driven by sunlight) produced when nitrogen<br />
oxides (NO x<br />
) react with carbon monoxide (CO),<br />
CH 4<br />
, non-methane hydrocarbons (NMHCs), and<br />
sunlight. Most of the pollutants which lead to<br />
the formation of tropospheric ozone in cities<br />
come from automobiles, power plants and other<br />
human activities. In addition to its long-term<br />
effects as a greenhouse gas, ozone is a major<br />
component of “smog,” which causes significant<br />
health problems for people in many cities,<br />
notably acute respiratory disorders. Ozone at<br />
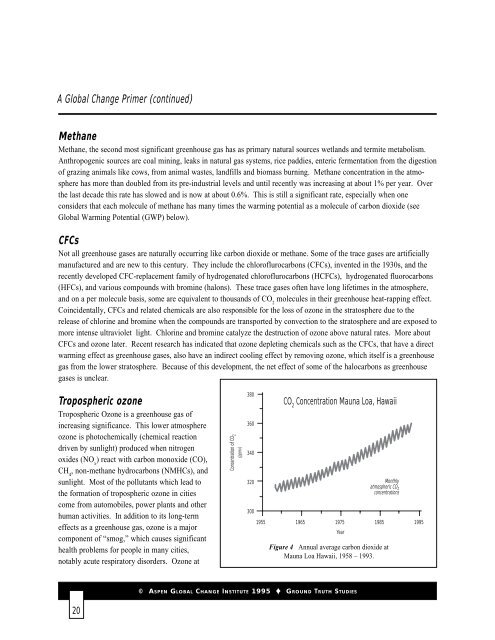
Concentration of CO 2<br />
(ppmv)<br />
380<br />
360<br />
340<br />
320<br />
CO 2<br />
Concentration Mauna Loa, Hawaii<br />
Monthly<br />
atmospheric CO 2<br />
concentrations<br />
300<br />
1955 1965 1975 1985 1995<br />
Year<br />
Figure 4 Annual average carbon dioxide at<br />
Mauna Loa Hawaii, 1958 – 1993.<br />
© ASPEN GLOBAL CHANGE INSTITUTE 1995 GROUND TRUTH STUDIES<br />
20










![View Powerpoint Slides [PDF]](https://img.yumpu.com/32486693/1/190x146/view-powerpoint-slides-pdf.jpg?quality=85)


![View Powerpoint Slides [PDF]](https://img.yumpu.com/29411106/1/190x143/view-powerpoint-slides-pdf.jpg?quality=85)

