DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
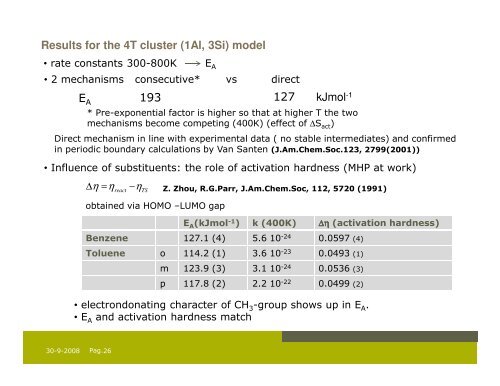
Results for the 4T cluster (1Al, 3Si) model<br />
• rate constants 300-800K E A<br />
• 2 mechanisms consecutive* vs direct<br />
E A<br />
193 127 kJmol -1<br />
* Pre-exponential factor is higher so that at higher T the two<br />
mechanisms become competing (400K) (effect of ∆S act )<br />
Direct mechanism in line with experimental data ( no stable intermediates) <strong>and</strong> confirmed<br />
in periodic boundary calculations by Van Santen (J.Am.Chem.Soc.123, 2799(2001))<br />
• Influence of substituents: the role of activation hardness (MHP at work)<br />
∆ η = η −η<br />
Z. Zhou, R.G.Parr, J.Am.Chem.Soc, 112, 5720 (1991)<br />
react<br />
TS<br />
obtained via HOMO –LUMO gap<br />
E A (kJmol -1 ) k (400K) ∆η (activation hardness)<br />
Benzene 127.1 (4) 5.6 10 -24 0.0597 (4)<br />
Toluene o 114.2 (1) 3.6 10 -23 0.0493 (1)<br />
m 123.9 (3) 3.1 10 -24 0.0536 (3)<br />
p 117.8 (2) 2.2 10 -22 0.0499 (2)<br />
• electrondonating character of CH 3 -group shows up in E A .<br />
• E A <strong>and</strong> activation hardness match<br />
Pag.<br />
30-9-2008 26