DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
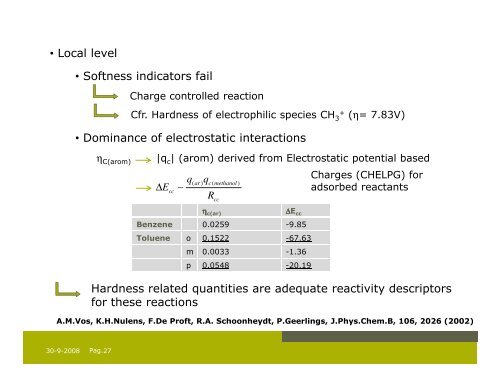
• Local level<br />
• Softness indicators fail<br />
Charge controlled reaction<br />
Cfr. Hardness of electrophilic species CH 3+ (η= 7.83V)<br />
• Dominance of electrostatic interactions<br />
η C(arom)<br />
|q c | (arom) derived from Electrostatic potential based<br />
∆E<br />
cc<br />
∼<br />
q<br />
q<br />
( ar ) c( methanol )<br />
R cc<br />
Charges (CHELPG) for<br />
adsorbed reactants<br />
η c(ar)<br />
∆E cc<br />
Benzene 0.0259 -9.85<br />
Toluene o 0.1522 -67.63<br />
m 0.0033 -1.36<br />
p 0.0548 -20.19<br />
Hardness related quantities are adequate reactivity descriptors<br />
for these reactions<br />
A.M.Vos, K.H.Nulens, F.De Proft, R.A. Schoonheydt, P.Geerlings, J.Phys.Chem.B, 106, 2026 (2002)<br />
Pag.<br />
30-9-2008 27