DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
DFT Reactivity Descriptors and Catalysis - Vrije Universiteit Brussel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
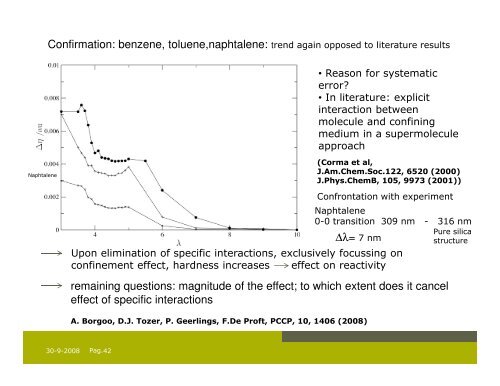
Confirmation: benzene, toluene,naphtalene: trend again opposed to literature results<br />
• Reason for systematic<br />
error?<br />
• In literature: explicit<br />
interaction between<br />
molecule <strong>and</strong> confining<br />
medium in a supermolecule<br />
approach<br />
Naphtalene<br />
(Corma et al,<br />
J.Am.Chem.Soc.122, 6520 (2000)<br />
J.Phys.ChemB, 105, 9973 (2001))<br />
Confrontation with experiment<br />
Upon elimination of specific interactions, exclusively focussing on<br />
confinement effect, hardness increases effect on reactivity<br />
remaining questions: magnitude of the effect; to which extent does it cancel<br />
effect of specific interactions<br />
A. Borgoo, D.J. Tozer, P. Geerlings, F.De Proft, PCCP, 10, 1406 (2008)<br />
Naphtalene<br />
0-0 transition 309 nm - 316 nm<br />
∆λ= 7 nm<br />
Pure silica<br />
structure<br />
Pag.<br />
30-9-2008 42