Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Cell Biology and Biophysics Unit<br />
Physical systems biochemistry of cytoskeleton<br />
dynamics and function<br />
Previous and current research<br />
The cytoskeleton is responsible for the internal organisation of eukaryotic cells. Microtubules,<br />
motor proteins and associated proteins form a mechano-chemical network that determines the dynamic<br />
and adaptable nature of intracellular order. But how the collective behaviour of various differently<br />
moving motors and competing regulators of microtubule dynamics leads to specific<br />
organisations of the cytoskeleton is not understood. How do single molecules move in cells? What<br />
role does spatio-temporal control of activities play in the correct functioning of motor/microtubule<br />
networks? Can we construct minimal systems in vitro that display complex network dynamics<br />
with defined functionalities? And does such a synthetic approach help us to understand<br />
what is special about the functioning of mechano-chemical systems distant from thermodynamic<br />
equilibrium?<br />
We address these questions using a combination<br />
of advanced light microscopy, biochemistry and<br />
quantitative cell biology. Our aim is to understand<br />
the behaviour of dynamic systems based on measured<br />
molecular properties. Therefore, we have<br />
studied how single fluorescently-labelled motors<br />
behave on single microtubules populated with competing molecules (Telley et al., 2009, Biophys.<br />
J.). We have measured the movements of motors in intact mitotic spindles and have investigated<br />
how the biophysical properties of an essential mitotic motor are regulated by a<br />
kinase in its physiological context. We believe that in vitro reconstitutions of dynamic cytoskeleton<br />
behaviour from a minimal set of dynamically interacting proteins is a powerful approach<br />
for the dissection of systems behaviour. Microtubule end-tracking and self-organisation<br />
of networks consisting of microtubules and different motors (Surrey et al., 2001, Science) are<br />
examples where system dynamics can be understood based on biochemical reconstitution<br />
combined with quantitative analysis.<br />
Future projects and goals<br />
Thomas Surrey<br />
PhD 1995, Eberhard-Karls<br />
University, Tübingen.<br />
Postdoctoral research at<br />
Princeton University, USA<br />
and <strong>EMBL</strong>.<br />
Staff Scientist 2001-2002.<br />
Team leader at <strong>EMBL</strong> since<br />
2002. Group leader since<br />
2006.<br />
In the future, we will continue to measure the biophysical properties of motors and microtubules<br />
both in their physiological context and in vitro, aiming at connecting single molecule<br />
physics with systems behaviour. We will develop tools that will allow us monitor and manipulate<br />
the spatio-temporal regulation of protein activities using chemical biology approaches in<br />
combination with advanced light microscopy. We will continue to generate more and more<br />
complex dynamic systems in vitro and to dissect their functions at a molecular level. Examples<br />
are microtubule end-tracking networks, mitotic spindles and cytoskeleton-membrane<br />
systems. Our goal is to understand how biological function of protein interaction networks is<br />
generated from the coordinated and regulated dynamic interactions of their components. In<br />
summary, we are interested in elucidating the design principles underlying intracellular organisation<br />
and dynamics using a combination of top-down and bottom-up approaches.<br />
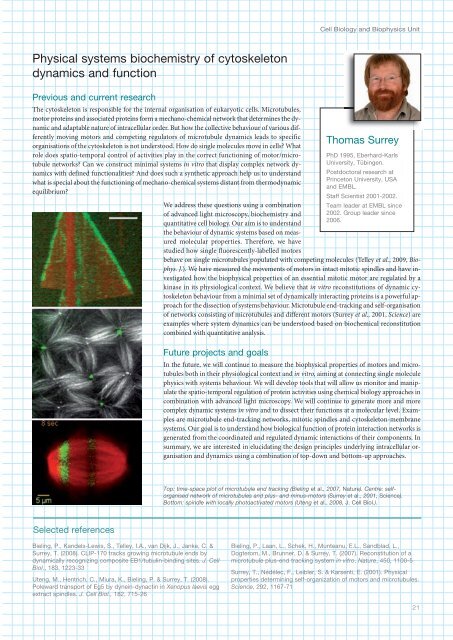
Top: time-space plot of microtubule end tracking (Bieling et al., 2007, Nature). Centre: selforganised<br />
network of microtubules and plus- and minus-motors (Surrey et al., 2001, Science).<br />
Bottom: spindle with locally photoactivated motors (Uteng et al., 2008, J. Cell Biol.).<br />
Selected references<br />
Bieling, P., Kandels-Lewis, S., Telley, I.A., van Dijk, J., Janke, C. &<br />
Surrey, T. (2008). CLIP-170 tracks growing microtubule ends by<br />
dynamically recognizing composite EB1/tubulin-binding sites. J. Cell<br />
Biol., 183, 1223-33<br />
Uteng, M., Hentrich, C., Miura, K., Bieling, P. & Surrey, T. (2008).<br />
Poleward transport of Eg5 by dynein-dynactin in Xenopus laevis egg<br />
extract spindles. J. Cell Biol., 182, 715-26<br />
Bieling, P., Laan, L., Schek, H., Munteanu, E.L., Sandblad, L.,<br />
Dogterom, M., Brunner, D. & Surrey, T. (2007). Reconstitution of a<br />
microtubule plus-end tracking system in vitro. Nature, 50, 1100-5<br />
Surrey, T., Nédélec, F., Leibler, S. & Karsenti, E. (2001). Physical<br />
properties determining self-organization of motors and microtubules.<br />
Science, 292, 1167-71<br />
21