Chem3D Users Manual - CambridgeSoft
Chem3D Users Manual - CambridgeSoft
Chem3D Users Manual - CambridgeSoft
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Administrator<br />
The procedures assume you have a basic<br />
understanding of the computational concepts and<br />
terminology of semi-empirical methods, and the<br />
concepts involved in geometry optimization<br />
(minimization) and single-point computations. For<br />
more information see “Computation Concepts” on<br />
page 129.<br />
For help with MOPAC, see the online MOPAC<br />
manual at:<br />
http://www.cachesoftware.com/mopac/Mopac2002<br />
manual/<br />
MOPAC Semiempirical<br />
Methods<br />
The method descriptions that follow represent a<br />
very simplified view of the semi-empirical methods<br />
available in <strong>Chem3D</strong> and CS MOPAC. For more<br />
information see the online MOPAC manual.<br />
Extended Hückel Method<br />
Developed from the qualitative Hückel MO<br />
method, the Extended Hückel Method (EH)<br />
represents the earliest one-electron semi-empirical<br />
method to incorporate both σ and p valence<br />
systems. It is still widely used, owing to its versatility<br />
and success in analyzing and interpreting groundstate<br />
properties of organic, organometallic, and<br />
inorganic compounds of biological interest. Built<br />
into <strong>Chem3D</strong>, EH is the default semi-empirical<br />
method used to calculate data required for<br />
displaying molecular surfaces.<br />
The EH method uses a one-electron Hamiltonian<br />
with matrix elements defined as follows:<br />
H µµ = – I µ<br />
H µν = 0.5K( H µµ + H νν )S µν µ ≠ ν<br />
where I µ is the valence state ionization energy<br />
(VSIE) of orbital µ as deduced from spectroscopic<br />
data, and K is the Wolfsberg-Helmholtz constant<br />
(usually taken as 1.75). The Hamiltonian neglects<br />
electron repulsion matrix elements but retains the<br />
overlap integrals calculated using Slater-type basis<br />
orbitals. Because the approximated Hamiltonian<br />
(H) does not depend on the MO expansion<br />
coefficient C νi , the matrix form of the EH<br />
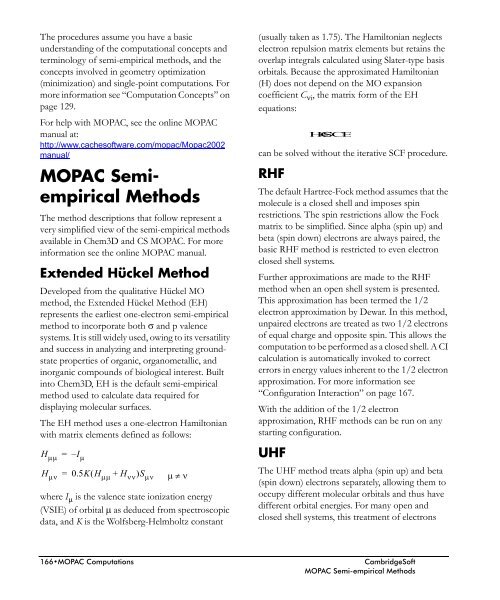
equations:<br />
can be solved without the iterative SCF procedure.<br />
RHF<br />
The default Hartree-Fock method assumes that the<br />
molecule is a closed shell and imposes spin<br />
restrictions. The spin restrictions allow the Fock<br />
matrix to be simplified. Since alpha (spin up) and<br />
beta (spin down) electrons are always paired, the<br />
basic RHF method is restricted to even electron<br />
closed shell systems.<br />
Further approximations are made to the RHF<br />
method when an open shell system is presented.<br />
This approximation has been termed the 1/2<br />
electron approximation by Dewar. In this method,<br />
unpaired electrons are treated as two 1/2 electrons<br />
of equal charge and opposite spin. This allows the<br />
computation to be performed as a closed shell. A CI<br />
calculation is automatically invoked to correct<br />
errors in energy values inherent to the 1/2 electron<br />
approximation. For more information see<br />
“Configuration Interaction” on page 167.<br />
With the addition of the 1/2 electron<br />
approximation, RHF methods can be run on any<br />
starting configuration.<br />
UHF<br />
HC=SCE<br />
The UHF method treats alpha (spin up) and beta<br />
(spin down) electrons separately, allowing them to<br />
occupy different molecular orbitals and thus have<br />
different orbital energies. For many open and<br />
closed shell systems, this treatment of electrons<br />
166•MOPAC Computations<br />
<strong>CambridgeSoft</strong><br />
MOPAC Semi-empirical Methods