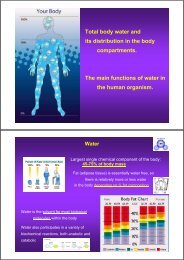
Fluid balance and electrolyte distribution in human body.
Fluid balance and electrolyte distribution in human body.
Fluid balance and electrolyte distribution in human body.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Calcium (Ca 2+ )<br />
– Most abundant ion <strong>in</strong> <strong>body</strong><br />
• plasma 2.3-2.6 mmol/L<br />
• most stored <strong>in</strong> bone (98%) as hydroxyapatite<br />
- Necessary for structure of bones <strong>and</strong> teeth, blood clott<strong>in</strong>g, hormone<br />
secretion, <strong>and</strong> cell receptor function<br />
- Regulation:<br />
• Parathyroid Hormone (PTH) - ↑ blood Ca 2+<br />
• Calciton<strong>in</strong> (CT) - ↓ blood Ca 2+<br />
- Homeostatic im<strong>balance</strong>s:<br />
Hypocalcemia (necrotic pancreatitis, malabsorption, hypoparathyreosis,<br />
vitam<strong>in</strong> D deficit (osteomalacia)) - muscle cramps, convulsions<br />
Therapy: 1. cause, 2. 10% Ca-gluconicum (10ml ampules, 1ml =<br />
0.25mmol)<br />
Hypercalcemia (hyperparathyreosis, hypervitam<strong>in</strong>osis D, osteolytic tumor<br />
metastasis)- vomit<strong>in</strong>g, cardiovascular symptoms, coma (critical [Ca+] is<br />
above 3.75 mmol/l); prolonged abnormal calcium deposition, e.g.,<br />
stone formation<br />
Therapy: 1.cause, 2. <strong>in</strong>crease of diuresis by furosemide (at 3L/day),<br />
glucocorticoids decreas<strong>in</strong>g Ca+ absorption by <strong>in</strong>test<strong>in</strong>e, calciton<strong>in</strong><br />
Phosphate (H 2 PO 4- , HPO 4<br />
2-<br />
, PO 4<br />
3-<br />
)<br />
– Important ICF anions; plasma 0.7-1.5 mmol/L<br />
• most (85%) is stored <strong>in</strong> bone as calcium salts<br />
• also comb<strong>in</strong>ed with lipids, prote<strong>in</strong>s, carbohydrates, nucleic acids<br />
(DNA <strong>and</strong> RNA), <strong>and</strong> high energy phosphate transport compound<br />
• important acid-base buffer <strong>in</strong> <strong>body</strong> fluids<br />
– Regulation - regulated <strong>in</strong> an <strong>in</strong>verse relationship with Ca 2+ by PTH <strong>and</strong><br />
calciton<strong>in</strong> <strong>and</strong> Vitam<strong>in</strong> D (If the concentration of one <strong>in</strong>creases, that of<br />
the other decreases)<br />
– Parathyroid hormone (PTH) - Increases plasma calcium levels<br />
– Vitam<strong>in</strong> D (fat-soluble steroid) - Increases calcium absorption from the<br />
GI tract<br />
– Calciton<strong>in</strong> - Decreases plasma calcium levels<br />
– Homeostatic im<strong>balance</strong>s<br />
• Phosphate concentrations shift oppositely from calcium<br />
concentrations <strong>and</strong> symptoms are usually due to the related<br />
calcium excess or deficit