April Journal-2009.p65 - Association of Biotechnology and Pharmacy
April Journal-2009.p65 - Association of Biotechnology and Pharmacy
April Journal-2009.p65 - Association of Biotechnology and Pharmacy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Current Trends in <strong>Biotechnology</strong> <strong>and</strong> <strong>Pharmacy</strong><br />
Vol. 3 (2) 113-127, <strong>April</strong> 2009. ISSN 0973-8916<br />
pr<strong>of</strong>essional to distribute <strong>and</strong> oversee vaccinations<br />
but these conditions are not required for edible<br />
vaccines. The goal is to give people in developing<br />
countries the genetically engineered seeds that<br />
will sprout edible vaccines. “Every culture on this<br />
planet raises food” explains Hein. “This can<br />
provide developing countries with a stable vaccine<br />
source because it will be genetically coded into<br />
the food”. Using recombinant DNA technology,<br />
researchers can now isolate the genes—called<br />
antigens—that mobilize our natural defences. But<br />
impregnating plants with these antigens requires<br />
an impressive bit <strong>of</strong> molecular legerdemain. At<br />
Scrips Research Institute, for instance, the antigen<br />
116<br />
is snipped <strong>of</strong>f the deadly cholera pathogen. Then<br />
it is inserted into the cells <strong>of</strong> a bacterium that<br />
causes a plant disease called crown gall. The<br />
alfalfa plants are infected with these transgenic<br />
crown gall organisms, which can penetrate the<br />
plant’s cell walls. The plant cells containing the<br />
foreign genes are then cultured in a Petri dish<br />
until they are mature enough to be transplanted.<br />
The next step is to test the potency <strong>of</strong> the antigens<br />
in plants raised in the field, outside <strong>of</strong> the cloistered<br />
laboratory. “We’ve just harvested this crop <strong>of</strong><br />
alfalfa” says Hein, who’s in the midst <strong>of</strong> measuring<br />
its antigen level <strong>and</strong> feed this transgenic grain to<br />
mice (64, 39 & 40, 24, 1,2 & 3, 48, 8, 21, 4). .<br />
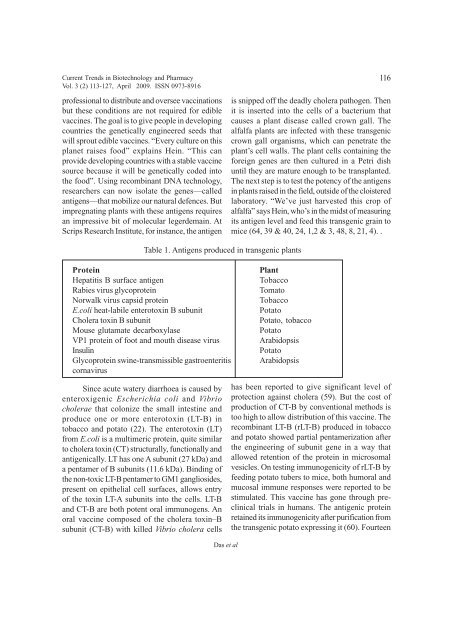
Table 1. Antigens produced in transgenic plants<br />
Protein<br />
Hepatitis B surface antigen<br />
Rabies virus glycoprotein<br />
Norwalk virus capsid protein<br />
E.coli heat-labile enterotoxin B subunit<br />
Cholera toxin B subunit<br />
Mouse glutamate decarboxylase<br />
VP1 protein <strong>of</strong> foot <strong>and</strong> mouth disease virus<br />
Insulin<br />
Glycoprotein swine-transmissible gastroenteritis<br />
cornavirus<br />
Plant<br />
Tobacco<br />
Tomato<br />
Tobacco<br />
Potato<br />
Potato, tobacco<br />
Potato<br />
Arabidopsis<br />
Potato<br />
Arabidopsis<br />
Since acute watery diarrhoea is caused by<br />
enteroxigenic Escherichia coli <strong>and</strong> Vibrio<br />
cholerae that colonize the small intestine <strong>and</strong><br />
produce one or more enterotoxin (LT-B) in<br />
tobacco <strong>and</strong> potato (22). The enterotoxin (LT)<br />
from E.coli is a multimeric protein, quite similar<br />
to cholera toxin (CT) structurally, functionally <strong>and</strong><br />
antigenically. LT has one A subunit (27 kDa) <strong>and</strong><br />
a pentamer <strong>of</strong> B subunits (11.6 kDa). Binding <strong>of</strong><br />
the non-toxic LT-B pentamer to GM1 gangliosides,<br />
present on epithelial cell surfaces, allows entry<br />
<strong>of</strong> the toxin LT-A subunits into the cells. LT-B<br />
<strong>and</strong> CT-B are both potent oral immunogens. An<br />
oral vaccine composed <strong>of</strong> the cholera toxin–B<br />
subunit (CT-B) with killed Vibrio cholera cells<br />
has been reported to give significant level <strong>of</strong><br />
protection against cholera (59). But the cost <strong>of</strong><br />
production <strong>of</strong> CT-B by conventional methods is<br />
too high to allow distribution <strong>of</strong> this vaccine. The<br />
recombinant LT-B (rLT-B) produced in tobacco<br />
<strong>and</strong> potato showed partial pentamerization after<br />
the engineering <strong>of</strong> subunit gene in a way that<br />
allowed retention <strong>of</strong> the protein in microsomal<br />
vesicles. On testing immunogenicity <strong>of</strong> rLT-B by<br />
feeding potato tubers to mice, both humoral <strong>and</strong><br />
mucosal immune responses were reported to be<br />
stimulated. This vaccine has gone through preclinical<br />
trials in humans. The antigenic protein<br />
retained its immunogenicity after purification from<br />
the transgenic potato expressing it (60). Fourteen<br />
Das et al