Dissolved organic matter in water of Daugava river
Dissolved organic matter in water of Daugava river
Dissolved organic matter in water of Daugava river
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
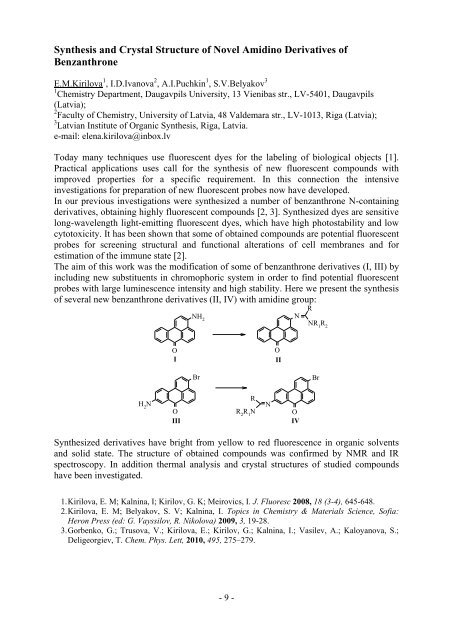
Synthesis and Crystal Structure <strong>of</strong> Novel Amid<strong>in</strong>o Derivatives <strong>of</strong>BenzanthroneE.M.Kirilova 1 , I.D.Ivanova 2 , A.I.Puchk<strong>in</strong> 1 , S.V.Belyakov 31 Chemistry Department, Daugavpils University, 13 Vienibas str., LV-5401, Daugavpils(Latvia);2 Faculty <strong>of</strong> Chemistry, University <strong>of</strong> Latvia, 48 Valdemara str., LV-1013, Riga (Latvia);3 Latvian Institute <strong>of</strong> Organic Synthesis, Riga, Latvia.e-mail: elena.kirilova@<strong>in</strong>box.lvToday many techniques use fluorescent dyes for the label<strong>in</strong>g <strong>of</strong> biological objects [1].Practical applications uses call for the synthesis <strong>of</strong> new fluorescent compounds withimproved properties for a specific requirement. In this connection the <strong>in</strong>tensive<strong>in</strong>vestigations for preparation <strong>of</strong> new fluorescent probes now have developed.In our previous <strong>in</strong>vestigations were synthesized a number <strong>of</strong> benzanthrone N-conta<strong>in</strong><strong>in</strong>gderivatives, obta<strong>in</strong><strong>in</strong>g highly fluorescent compounds [2, 3]. Synthesized dyes are sensitivelong-wavelength light-emitt<strong>in</strong>g fluorescent dyes, which have high photostability and lowcytotoxicity. It has been shown that some <strong>of</strong> obta<strong>in</strong>ed compounds are potential fluorescentprobes for screen<strong>in</strong>g structural and functional alterations <strong>of</strong> cell membranes and forestimation <strong>of</strong> the immune state [2].The aim <strong>of</strong> this work was the modification <strong>of</strong> some <strong>of</strong> benzanthrone derivatives (I, III) by<strong>in</strong>clud<strong>in</strong>g new substituents <strong>in</strong> chromophoric system <strong>in</strong> order to f<strong>in</strong>d potential fluorescentprobes with large lum<strong>in</strong>escence <strong>in</strong>tensity and high stability. Here we present the synthesis<strong>of</strong> several new benzanthrone derivatives (II, IV) with amid<strong>in</strong>e group:NH 2NRNR 1R 2OIOIIBrBrN H 2OIIIRR 2R 1NNOIVSynthesized derivatives have bright from yellow to red fluorescence <strong>in</strong> <strong>organic</strong> solventsand solid state. The structure <strong>of</strong> obta<strong>in</strong>ed compounds was confirmed by NMR and IRspectroscopy. In addition thermal analysis and crystal structures <strong>of</strong> studied compoundshave been <strong>in</strong>vestigated.1. Kirilova, E. M; Kaln<strong>in</strong>a, I; Kirilov, G. K; Meirovics, I. J. Fluoresc 2008, 18 (3-4), 645-648.2. Kirilova, E. M; Belyakov, S. V; Kaln<strong>in</strong>a, I. Topics <strong>in</strong> Chemistry & Materials Science, S<strong>of</strong>ia:Heron Press (ed: G. Vayssilov, R. Nikolova) 2009, 3, 19-28.3. Gorbenko, G.; Trusova, V.; Kirilova, E.; Kirilov, G.; Kaln<strong>in</strong>a, I.; Vasilev, A.; Kaloyanova, S.;Deligeorgiev, T. Chem. Phys. Lett, 2010, 495, 275–279.- 9 -