- Page 2 and 3:
Peptide-Based Drug Design
- Page 4 and 5:
METHODS IN MOLECULAR BIOLOGY TM Pep
- Page 6 and 7:
Preface Natural products chemistry
- Page 8 and 9:
Contents Preface...................
- Page 10 and 11:
Contributors Nikolinka Antcheva •
- Page 12 and 13:
Contributors xi Alessandro Tossi
- Page 14 and 15:
2 Otvos advance of computer power a
- Page 16 and 17:
4 Otvos see derivatives active in b
- Page 18 and 19:
6 Otvos was designed based on ligan
- Page 20 and 21:
8 Otvos 27. Borghouts, C., Kunz, C.
- Page 22 and 23:
10 Bulet Key Words: Invertebrate im
- Page 24 and 25:
12 Bulet 6. 5 �L or higher volume
- Page 26 and 27:
14 Bulet 2.5.2.1. MALDI-TOF-MS 1. M
- Page 28 and 29:
16 Bulet 7. Small-volume low-protei
- Page 30 and 31:
18 Bulet 3. Centrifuge between 8000
- Page 32 and 33:
20 Bulet internal diameter). Increa
- Page 34 and 35:
22 Bulet interest. As no instrument
- Page 36 and 37:
24 Bulet 3. Incubate the plates in
- Page 38 and 39:
26 Bulet bioactive peptides from th
- Page 40 and 41:
28 Bulet 21. Chernysh, S., Kim, S.I
- Page 42 and 43:
3 Sequence Analysis of Antimicrobia
- Page 44 and 45:
Sequence Analysis of Antimicrobial
- Page 46 and 47:
Sequence Analysis of Antimicrobial
- Page 48 and 49:
Sequence Analysis of Antimicrobial
- Page 50 and 51:
Sequence Analysis of Antimicrobial
- Page 52 and 53:
Sequence Analysis of Antimicrobial
- Page 54 and 55:
Sequence Analysis of Antimicrobial
- Page 56 and 57:
Sequence Analysis of Antimicrobial
- Page 58 and 59:
4 The Spot Technique: Synthesis and
- Page 60 and 61:
The Spot Technique 49 3. For amine
- Page 62 and 63:
The Spot Technique 51 2. Biotinylat
- Page 64 and 65:
The Spot Technique 53 the solutions
- Page 66 and 67:
The Spot Technique 55 solution. Ens
- Page 68 and 69:
The Spot Technique 57 (see Fig. 3)
- Page 70 and 71:
The Spot Technique 59 Fig. 5. Princ
- Page 72 and 73:
The Spot Technique 61 library, the
- Page 74 and 75:
The Spot Technique 63 7. Regenerati
- Page 76 and 77:
The Spot Technique 65 following rul
- Page 78 and 79:
The Spot Technique 67 20. Atherton,
- Page 80 and 81:
The Spot Technique 69 50. Bolger, G
- Page 82 and 83:
5 Analysis of A� Interactions Usi
- Page 84 and 85:
Analysis of Aβ Interactions 73 are
- Page 86 and 87:
Analysis of Aβ Interactions 75 Ana
- Page 88 and 89:
Analysis of Aβ Interactions 77 3.
- Page 90 and 91:
Analysis of Aβ Interactions 79 4.
- Page 92 and 93:
Analysis of Aβ Interactions 81 5.
- Page 94 and 95:
Analysis of Aβ Interactions 83 2.
- Page 96 and 97:
Analysis of Aβ Interactions 85 9.
- Page 98 and 99:
6 NMR in Peptide Drug Development J
- Page 100 and 101:
NMR of Peptides 89 usually require
- Page 102 and 103:
NMR of Peptides 91 limiting the siz
- Page 104 and 105:
NMR of Peptides 93 and STD NMR expe
- Page 106 and 107:
NMR of Peptides 95 The basis of the
- Page 108 and 109:
NMR of Peptides 97 Fig. 5. Overlay
- Page 110 and 111:
NMR of Peptides 99 Fig. 7. Schemati
- Page 112 and 113:
NMR of Peptides 101 4.4.2. Saturati
- Page 114 and 115:
NMR of Peptides 103 4.6. Diffusion
- Page 116 and 117:
NMR of Peptides 105 Fig. 13. Propos
- Page 118 and 119:
NMR of Peptides 107 protein prevent
- Page 120 and 121:
NMR of Peptides 109 6. Wuthrich, K.
- Page 122 and 123:
NMR of Peptides 111 45. Morris, K.
- Page 124 and 125:
NMR of Peptides 113 78. Aumailley,
- Page 126 and 127:
116 Copps et al. Fig. 1. Potential
- Page 128 and 129:
118 Copps et al. Fig. 2. Flowchart
- Page 130 and 131:
120 Copps et al. 10. In accordance
- Page 132 and 133:
122 Copps et al. Fig. 3. DSSP for g
- Page 134 and 135:
124 Copps et al. ingly with the sam
- Page 136 and 137:
126 Copps et al. 19. Essman, U., Pe
- Page 138 and 139:
128 Hilpert et al. Key Words: Scree
- Page 140 and 141:
130 Hilpert et al. Table 1 (Continu
- Page 142 and 143:
132 Hilpert et al. different natura
- Page 144 and 145:
134 Hilpert et al. wt A C D E F …
- Page 146 and 147:
136 Hilpert et al. methods primaril
- Page 148 and 149:
138 Hilpert et al. Table 2 (Continu
- Page 150 and 151:
140 Hilpert et al. Table 2 (Continu
- Page 152 and 153:
142 Hilpert et al. Table 2 (Continu
- Page 154 and 155: 144 Hilpert et al. Table 3 Summary
- Page 156 and 157: 146 Hilpert et al. Table 3 (Continu
- Page 158 and 159: 148 Hilpert et al. of substituting
- Page 160 and 161: 150 Hilpert et al. in models that c
- Page 162 and 163: 152 Hilpert et al. than control. Gi
- Page 164 and 165: 154 Hilpert et al. Table 4 Accuracy
- Page 166 and 167: 156 Hilpert et al. 25. Pini, A., Gi
- Page 168 and 169: 158 Hilpert et al. 55. Giacometti,
- Page 170 and 171: 9 Investigating the Mode of Action
- Page 172 and 173: Proline-Rich Antimicrobial Peptides
- Page 174 and 175: Proline-Rich Antimicrobial Peptides
- Page 176 and 177: Proline-Rich Antimicrobial Peptides
- Page 178 and 179: Proline-Rich Antimicrobial Peptides
- Page 180 and 181: Proline-Rich Antimicrobial Peptides
- Page 182 and 183: Proline-Rich Antimicrobial Peptides
- Page 184 and 185: Proline-Rich Antimicrobial Peptides
- Page 186 and 187: 10 Serum Stability of Peptides Håv
- Page 188 and 189: Serum Stability of Peptides 179 2.
- Page 190 and 191: Serum Stability of Peptides 181 9.
- Page 192 and 193: Serum Stability of Peptides 183 �
- Page 194 and 195: Serum Stability of Peptides 185 13.
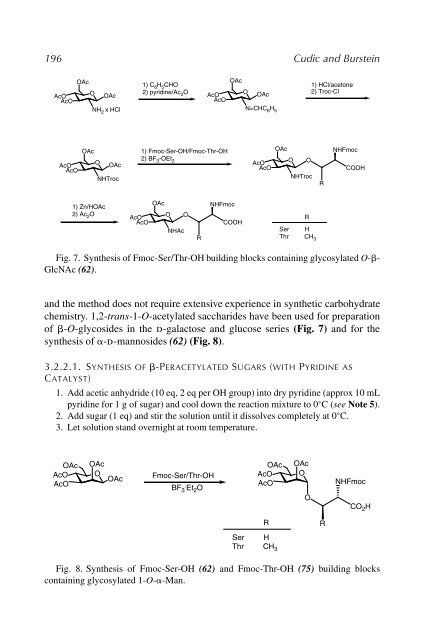
- Page 196 and 197: 11 Preparation of Glycosylated Amin
- Page 198 and 199: Glycosylated Amino Acid Synthesis 1
- Page 200 and 201: Glycosylated Amino Acid Synthesis 1
- Page 202 and 203: Glycosylated Amino Acid Synthesis 1
- Page 206 and 207: Glycosylated Amino Acid Synthesis 1
- Page 208 and 209: Glycosylated Amino Acid Synthesis 1
- Page 210 and 211: Glycosylated Amino Acid Synthesis 2
- Page 212 and 213: Glycosylated Amino Acid Synthesis 2
- Page 214 and 215: Glycosylated Amino Acid Synthesis 2
- Page 216 and 217: Glycosylated Amino Acid Synthesis 2
- Page 218 and 219: 12 Synthesis of O-Phosphopeptides o
- Page 220 and 221: Solid-Phase Synthesis of O-Phosphop
- Page 222 and 223: Solid-Phase Synthesis of O-Phosphop
- Page 224 and 225: Solid-Phase Synthesis of O-Phosphop
- Page 226 and 227: Solid-Phase Synthesis of O-Phosphop
- Page 228 and 229: Solid-Phase Synthesis of O-Phosphop
- Page 230 and 231: Solid-Phase Synthesis of O-Phosphop
- Page 232 and 233: 13 Peptidomimetics: Fmoc Solid-Phas
- Page 234 and 235: Peptidomimetics 225 O O R S N H Pep
- Page 236 and 237: Peptidomimetics 227 not without its
- Page 238 and 239: Peptidomimetics 229 Oxidation step:
- Page 240 and 241: Peptidomimetics 231 of peptidosulfo
- Page 242 and 243: Peptidomimetics 233 8. Allow the re
- Page 244 and 245: Peptidomimetics 235 3.3.2. Solid-Ph
- Page 246 and 247: Peptidomimetics 237 On the other ha
- Page 248 and 249: Peptidomimetics 239 nitrogen (73).
- Page 250 and 251: Peptidomimetics 241 3. Cover the re
- Page 252 and 253: Peptidomimetics 243 efficient synth
- Page 254 and 255:
Peptidomimetics 245 55. Alsina, J.,
- Page 256 and 257:
14 Synthesis of Toll-Like Receptor-
- Page 258 and 259:
Lipopeptides 249 2. Materials 2.1.
- Page 260 and 261:
Lipopeptides 251 Fig. 1. Structural
- Page 262 and 263:
Lipopeptides 253 8. To enable lipid
- Page 264 and 265:
Lipopeptides 255 Representative res
- Page 266 and 267:
Lipopeptides 257 Fig. 2. Immunogeni
- Page 268 and 269:
Lipopeptides 259 8. A large plastic
- Page 270 and 271:
Lipopeptides 261 26. Nardin, E.H.,
- Page 272 and 273:
264 Otvos response, synthetic pepti
- Page 274 and 275:
266 Otvos Fig. 3. Schematic present
- Page 276 and 277:
268 Otvos 3. Methods The main goal
- Page 278 and 279:
270 Otvos 3.2.3. Purification and Q
- Page 280 and 281:
272 Otvos 6. Meyer, D., and Torres,
- Page 282 and 283:
16 Cysteine-Containing Fusion Tag f
- Page 284 and 285:
Targeted Therapeutic and Imaging Ag
- Page 286 and 287:
Targeted Therapeutic and Imaging Ag
- Page 288 and 289:
Targeted Therapeutic and Imaging Ag
- Page 290 and 291:
Targeted Therapeutic and Imaging Ag
- Page 292 and 293:
Targeted Therapeutic and Imaging Ag
- Page 294 and 295:
Targeted Therapeutic and Imaging Ag
- Page 296 and 297:
Targeted Therapeutic and Imaging Ag
- Page 298 and 299:
Targeted Therapeutic and Imaging Ag
- Page 300 and 301:
Targeted Therapeutic and Imaging Ag
- Page 302 and 303:
Index A A� peptides aggregation a
- Page 304 and 305:
Index 297 phosphopeptides influenci
- Page 306 and 307:
Index 299 for genetically synthetic
- Page 308 and 309:
Index 301 peptidosulfonamide, disad
- Page 310:
Index 303 solid phase, 180, 214 sul