<strong>Tour</strong>-<strong>de</strong>-<strong>Force</strong>: Interplay between Mitochondria and Cell Cycle Progression Fall 2007damage in the critical phase of DNA replication induced by Reactive Oxygen Species (ROS) (Brand et al.,1997). Glycolysis is increased during cell cycle progression, reaching a peak in the S phase. This peak ischaracterized by up-regulation of glycolytic enzyme activity and increased lactate production. Inproliferating cells, 86% of ATP is <strong>de</strong>rived from glucose breakdown to pyruvate and lactate (Brand et al.,1997). This rate of glycolysis is much higher than that in differentiated cells.In ischemic myocardium (a condition in which the heart muscle tissue is <strong>de</strong>prived from oxygen),activated AMPK has been shown to stimulate glycolysis by activating Phosphofructokinase 2 (PFK2),which is an upstream regulator of PFK1. PFK1 is a major glycolytic enzyme that has been found to be upregulateddirectly by AMP. It catalyses the most regulated step of glycolysis: the conversion of fructose 6-phosphate and ATP to fructose 1,6-biphosphate and ADP (Hue et al., 2003). In addition, AMPK is knownto activate glycolysis in skeletal muscle cells in response to hypoxia (<strong>de</strong>pletion of oxygen) or musclecontraction, for which much energy is nee<strong>de</strong>d (Hue et al., 2007). Chronic or acute AMPK activation inskeletal muscle increases GLUT4 and GLUT1 expression, which results in increased glucose uptake (Hueet al., 2003). Lastly, changes in AMPK activity are shown to regulate glucose production in differentiatedliver cells as well (Viollet et al., 2006).We suggest that AMPK activation in G1 will induce glycolysis (among other metabolic processes) inor<strong>de</strong>r to re-establish the energetic balance that will enable the cell to progress through the cell cycle. Thisresearch aims to draw a more complete picture of the influence of AMPK on glycolysis in normalproliferating cells and in relation to AMPK-induced cell cycle arrest.Fatty acid oxidation (FAO) and AMPKIn addition to its role in glycolysis, AMPK is known to regulate FAO. In its active form, it increases bothprocesses in response to energy <strong>de</strong>mand induced by ischaemia or hypoxia (Dyck and Lopaschuk, 2006).However, in the absence of those conditions, the effects of AMPK activity on fatty acid oxidation areunknown. In many tissues, such as heart cells, the rate of fatty acid oxidation <strong>de</strong>pends on the plasmaconcentration and transport of fatty acids (Dyck and Lopaschuk, 2006). Also in heart tissue, AMPK playsan important role in regulating Malonyl CoA levels. Malonyl CoA inhibits CPT1, which is a mitochondrialouter membrane enzyme that is involved in transport of fatty acids into the mitochondria for oxidation. It issuggested that both acetyl CoA carboxylase (ACC) and malonyl CoA <strong>de</strong>carboxylase (MCD), whichinfluence malonyl CoA levels, are un<strong>de</strong>r direct phosphorylation control of AMPK (Dyck and Lopaschuk,2006). In addition, some studies <strong>de</strong>monstrate that activation of AMPK participates in the contractioninducedfatty acid oxidation in muscle cells (Suzuki et al., 2007). We suspect that AMPK activation willresult in increased fatty acid oxidation, possibly via the interaction with the enzymes mentioned above.AMPK as a regulator of the cell cycleBesi<strong>de</strong>s multiple effects of active AMPK on the cell's metabolism, it induces cell cycle arrest in G1 andpossibly apoptosis in proliferating cells (Jones et al., 2005; Tzatsos et al., 2007). AMPK inducesphosphorylation of p53 at a specific seronine residue, Ser-15. When p53 is phosphorylated at Ser-15 it isnot targeted for <strong>de</strong>gradation anymore and stabilizes within the cell. P53 subsequently up-regulatestranscription of p21 which is thought to bind and inhibit the cyclinE/Cdk2 complex (Mandal et al., 2005).P21 inhibits the activity of cyclinE/Cdk2 which when active, phosphorylates Rb, inducing E2F genetranscription nee<strong>de</strong>d for entry into S-phase. Thus, by stabilizing p53 in the cell, AMPK induces cell cyclearrest.Previous research has <strong>de</strong>monstrated significant changes in the levels of a number of proteins when cellsenter quiescence. Specifically levels of cyclin D1 as well as cyclin B exhibit a salient <strong>de</strong>crease upon entryinto the quiescent state (Pajalunga et al., 2007; Huss et al., 2000). Furthermore, the normal build up ofcyclin A towards the end of G1 is suppressed during quiescence. In addition, P130, which is a pocketfamily member protein of Rb, has been shown to be highly expressed in quiescent cells, whereas Rb itselfis mainly present in its unphosphorylated form (Blomen and Boonstra, 2007).The PI3-Kinase / Akt pathway has been postulated to be a central mediator of progression through G1. Ithas been <strong>de</strong>monstrated that inhibition of the PI3-kinase pathway shortly after or during mitosis leads toirreversible cell cycle arrest in early G1, followed by apoptosis (Hulleman, et al., 2004).SCI 332 Advanced Molecular Cell Biology Research Proposal 40
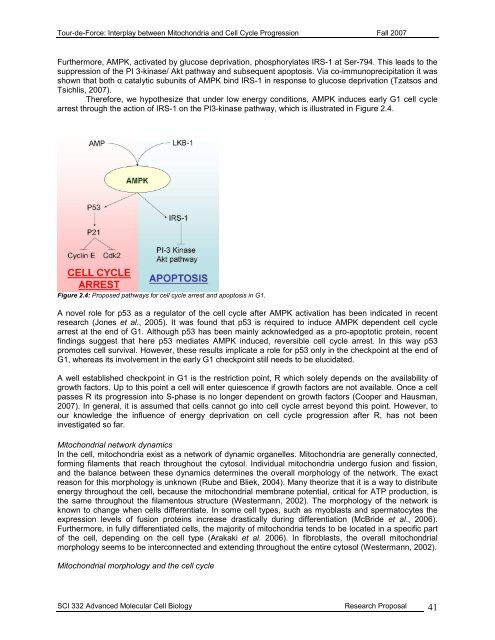
<strong>Tour</strong>-<strong>de</strong>-<strong>Force</strong>: Interplay between Mitochondria and Cell Cycle Progression Fall 2007Furthermore, AMPK, activated by glucose <strong>de</strong>privation, phosphorylates IRS-1 at Ser-794. This leads to thesuppression of the PI 3-kinase/ Akt pathway and subsequent apoptosis. Via co-immunoprecipitation it wasshown that both α catalytic subunits of AMPK bind IRS-1 in response to glucose <strong>de</strong>privation (Tzatsos andTsichlis, 2007).Therefore, we hypothesize that un<strong>de</strong>r low energy conditions, AMPK induces early G1 cell cyclearrest through the action of IRS-1 on the PI3-kinase pathway, which is illustrated in Figure 2.4.Figure 2.4: Proposed pathways for cell cycle arrest and apoptosis in G1.A novel role for p53 as a regulator of the cell cycle after AMPK activation has been indicated in recentresearch (Jones et al., 2005). It was found that p53 is required to induce AMPK <strong>de</strong>pen<strong>de</strong>nt cell cyclearrest at the end of G1. Although p53 has been mainly acknowledged as a pro-apoptotic protein, recentfindings suggest that here p53 mediates AMPK induced, reversible cell cycle arrest. In this way p53promotes cell survival. However, these results implicate a role for p53 only in the checkpoint at the end ofG1, whereas its involvement in the early G1 checkpoint still needs to be elucidated.A well established checkpoint in G1 is the restriction point, R which solely <strong>de</strong>pends on the availability ofgrowth factors. Up to this point a cell will enter quiescence if growth factors are not available. Once a cellpasses R its progression into S-phase is no longer <strong>de</strong>pen<strong>de</strong>nt on growth factors (Cooper and Hausman,2007). In general, it is assumed that cells cannot go into cell cycle arrest beyond this point. However, toour knowledge the influence of energy <strong>de</strong>privation on cell cycle progression after R, has not beeninvestigated so far.Mitochondrial network dynamicsIn the cell, mitochondria exist as a network of dynamic organelles. Mitochondria are generally connected,forming filaments that reach throughout the cytosol. Individual mitochondria un<strong>de</strong>rgo fusion and fission,and the balance between these dynamics <strong>de</strong>termines the overall morphology of the network. The exactreason for this morphology is unknown (Rube and Bliek, 2004). Many theorize that it is a way to distributeenergy throughout the cell, because the mitochondrial membrane potential, critical for ATP production, isthe same throughout the filamentous structure (Westermann, 2002). The morphology of the network isknown to change when cells differentiate. In some cell types, such as myoblasts and spermatocytes theexpression levels of fusion proteins increase drastically during differentiation (McBri<strong>de</strong> et al., 2006).Furthermore, in fully differentiated cells, the majority of mitochondria tends to be located in a specific partof the cell, <strong>de</strong>pending on the cell type (Arakaki et al. 2006). In fibroblasts, the overall mitochondrialmorphology seems to be interconnected and extending throughout the entire cytosol (Westermann, 2002).Mitochondrial morphology and the cell cycleSCI 332 Advanced Molecular Cell Biology Research Proposal 41