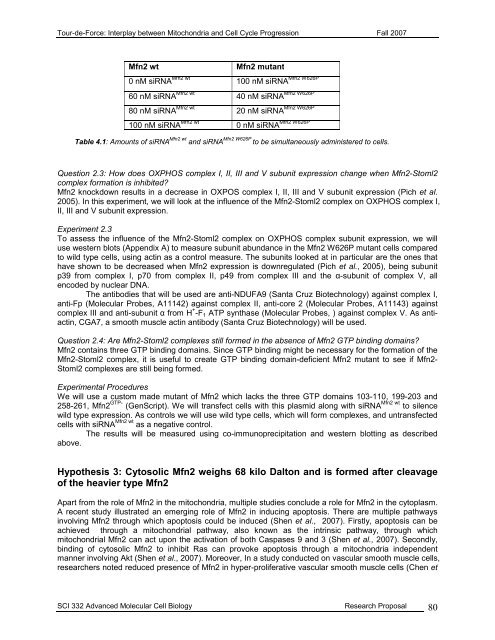
<strong>Tour</strong>-<strong>de</strong>-<strong>Force</strong>: Interplay between Mitochondria and Cell Cycle Progression Fall 2007Mfn2 wtMfn2 mutant0 nM siRNA Mfn2 wt Mfn2 W626P100 nM siRNA60 nM siRNA Mfn2 wt Mfn2 W626P40 nM siRNA80 nM siRNA Mfn2 wt Mfn2 W626P20 nM siRNA100 nM siRNA Mfn2 wt Mfn2 W626P0 nM siRNATable 4.1: Amounts of siRNA Mfn2 wt and siRNA Mfn2 W626P to be simultaneously administered to cells.Question 2.3: How does OXPHOS complex I, II, III and V subunit expression change when Mfn2-Stoml2complex formation is inhibited?Mfn2 knockdown results in a <strong>de</strong>crease in OXPOS complex I, II, III and V subunit expression (Pich et al.2005). In this experiment, we will look at the influence of the Mfn2-Stoml2 complex on OXPHOS complex I,II, III and V subunit expression.Experiment 2.3To assess the influence of the Mfn2-Stoml2 complex on OXPHOS complex subunit expression, we willuse western blots (Appendix A) to measure subunit abundance in the Mfn2 W626P mutant cells comparedto wild type cells, using actin as a control measure. The subunits looked at in particular are the ones thathave shown to be <strong>de</strong>creased when Mfn2 expression is downregulated (Pich et al., 2005), being subunitp39 from complex I, p70 from complex II, p49 from complex III and the α-subunit of complex V, allenco<strong>de</strong>d by nuclear DNA.The antibodies that will be used are anti-NDUFA9 (Santa Cruz Biotechnology) against complex I,anti-Fp (Molecular Probes, A11142) against complex II, anti-core 2 (Molecular Probes, A11143) againstcomplex III and anti-subunit α from H + -F 1 ATP synthase (Molecular Probes, ) against complex V. As antiactin,CGA7, a smooth muscle actin antibody (Santa Cruz Biotechnology) will be used.Question 2.4: Are Mfn2-Stoml2 complexes still formed in the absence of Mfn2 GTP binding domains?Mfn2 contains three GTP binding domains. Since GTP binding might be necessary for the formation of theMfn2-Stoml2 complex, it is useful to create GTP binding domain-<strong>de</strong>ficient Mfn2 mutant to see if Mfn2-Stoml2 complexes are still being formed.Experimental ProceduresWe will use a custom ma<strong>de</strong> mutant of Mfn2 which lacks the three GTP domains 103-110, 199-203 and258-261, Mfn2 GTP- (GenScript). We will transfect cells with this plasmid along with siRNA Mfn2 wt to silencewild type expression. As controls we will use wild type cells, which will form complexes, and untransfectedcells with siRNA Mfn2 wt as a negative control.The results will be measured using co-immunoprecipitation and western blotting as <strong>de</strong>scribedabove.Hypothesis 3: Cytosolic Mfn2 weighs 68 kilo Dalton and is formed after cleavageof the heavier type Mfn2Apart from the role of Mfn2 in the mitochondria, multiple studies conclu<strong>de</strong> a role for Mfn2 in the cytoplasm.A recent study illustrated an emerging role of Mfn2 in inducing apoptosis. There are multiple pathwaysinvolving Mfn2 through which apoptosis could be induced (Shen et al., 2007). Firstly, apoptosis can beachieved through a mitochondrial pathway, also known as the intrinsic pathway, through whichmitochondrial Mfn2 can act upon the activation of both Caspases 9 and 3 (Shen et al., 2007). Secondly,binding of cytosolic Mfn2 to inhibit Ras can provoke apoptosis through a mitochondria in<strong>de</strong>pen<strong>de</strong>ntmanner involving Akt (Shen et al., 2007). Moreover, In a study conducted on vascular smooth muscle cells,researchers noted reduced presence of Mfn2 in hyper-proliferative vascular smooth muscle cells (Chen etSCI 332 Advanced Molecular Cell Biology Research Proposal 80
<strong>Tour</strong>-<strong>de</strong>-<strong>Force</strong>: Interplay between Mitochondria and Cell Cycle Progression Fall 2007al., 2004). In this study, Mfn2 has been shown to be able to induce cell cycle arrest via inhibition of Ras,after which the Raf-MEK-ERK pathway is blocked.From this information, it seems viable to assume that Mfn2 plays a major role in the induction ofcell cycle arrest and apoptosis. In most findings it seems that it is specifically Mfn2 located in the cytosol,as opposed to mitochondrial Mfn2, that plays a role in these cell-progression pathways. Therefore, anexploration of the presence and formation of this cytosolic Mfn2 type is a logical step in our research, as itwill allow a more in <strong>de</strong>pth exploration of the regulative role of Mfn2 on the cell cycle.It should be noted that at first sight, Mfn2 seems to have contradicting roles within the cell. On onehand Mfn2 maintains OXPHOS, whereas on the other hand, Mfn2 can induce cell cycle arrest andapoptosis. This might be explained through a mechanism that cleaves mitochondrial Mfn-2 in case cellcycle arrest or apoptosis is nee<strong>de</strong>d. Through cleavage, mitochondrial Mfn2 levels will <strong>de</strong>crease, afterwhich OXPHOS maintenance will drop and energy levels will be lowered. As has been discussed inproject 1, this might lead to cell cycle arrest. On the other hand, cytosolic Mfn2 will increase, which caninduce cell cycle arrest or apoptosis.Question 3.1: Does cytosolic Mfn2 weigh 68 kD?In the research on hyper-proliferating vascular smooth muscle cells (Chen et al., 2004) it was discoveredthrough western blot analysis that two forms of Mfn2 were present in the cell. The two strands representedMfn2 types of a varying molecular weight. The first strand with a molecular weight of 86 kD was equallypresent in normal and highly proliferating cells. Contrary to the former, the second type of Mfn2, weighing68 kD, showed to be less present in hyper-proliferative cells. (Chen et al., 2004) This suggests a specificfunction for the lighter type Mfn2 in Ras inhibition. As Ras is inhibited by cytosolic Mfn2 it is viable tohypothesize that light-type Mfn2 represents in fact cytosolic Mfn2.In or<strong>de</strong>r to answer the question whether the 68 kD Mfn2 is restricted to the cytosol and the 86 kDMfn2 is restricted to the mitochondria, cell fractionation (Appendix A) and subsequently western analysis(Appendix A) will be used. The vascular smooth muscle cell culture HAoSMC-c (Promocell) will again beused.Experiment 3.1Fractionation will be execute<strong>de</strong>d by usage of a cytosol/mitochondria cell fractionation kit (BioVision). Thiskit separates the mitochondria from the cytosol, the latter will contain everything that is usually present inthe cytosol, except for the mitochondria. The procedure will be carried out as <strong>de</strong>scribed in themanufacturer’s protocol. Subsequently, western analysis will be exerted with help of a Western BreezeChemiluminescence Kit anti-goat (Invitrogen). The kit contains a secondary anti-goat IgG antibody(starting dilution 1:5000) conjugated with AP and an AP substrate. The procedure will be carried outaccording to the manufacturer’s manual.After fractionation, the cytosolic sample will be tested through western analysis for a protein thatis exclusively found in mitochondria, namely the protein Tom40. A primary antibody (starting dilution 1:200)from goat (Santa Cruz Biotechnology) will be obtained. The mitochondrial sample will be tested for aprotein that is only found in the cytosol as well. In this case the ribosomal protein L28 will be used. Again,a primary antibody (starting dilution 1:200) from goat (Santa Cruz Biotechnology) will be obtained. If bothtests are found to be negative, the fractionation has been successful. As an internal control for all westernblots (except for the mitochondrial samples) β-tubulin will be checked with help of a primary antibody β-tubulin L15 (Santa Cruz Biotechnology).Thereafter, the mitochondrial and cytosolic samples will be tested separately for Mfn2 throughwestern analysis. In or<strong>de</strong>r to <strong>de</strong>tect Mfn2 we will use the primary antibody Y-19 (Santa CruzBiotechnology), a goat polyclonal IgG antibody specific for the N-terminal (starting dilution 1:200) After thistest it can be conclu<strong>de</strong>d which weight-types of Mfn2 are present in both the mitochondria and the cytosol.Question 3.2: Is cytosolic Mfn2 formed after cleavage of the heavier type Mfn2?The structure of mitochondrial Mfn2 is known and is schematically represented in Figure 4.1 (inintroduction). Mfn2 consists out of 757 amino acids. The first 604 amino acids account for the N-terminalcytosolic part of Mfn2, containing both a GTPase and a heptad repeat region, which forms a coiled coil.Amino acids 605-625 and 627-647 account for the trans-membrane regions (TMD). Furthermore, aminoacids 648-757 account for the C-terminal cytosolic region, containing heptad repeat region and a coiledcoil (Swissprot).SCI 332 Advanced Molecular Cell Biology Research Proposal 81