FINISHED_Final_Notebook_Jones
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
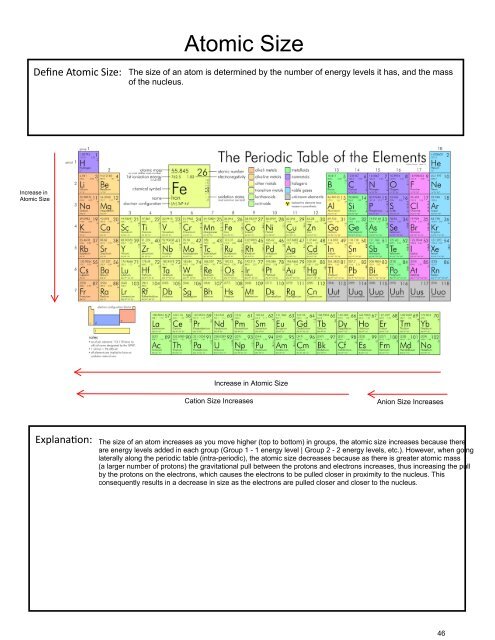
Atomic Size<br />
Define Atomic Size: The size of an atom is determined by the number of energy levels it has, and the mass<br />
of the nucleus.<br />
Increase in<br />
Atomic Size<br />
Increase in Atomic Size<br />
Cation Size Increases<br />
Anion Size Increases<br />
Explanation:<br />
The size of an atom increases as you move higher (top to bottom) in groups, the atomic size increases because there<br />
are energy levels added in each group (Group 1 - 1 energy level | Group 2 - 2 energy levels, etc.). However, when going<br />
laterally along the periodic table (intra-periodic), the atomic size decreases because as there is greater atomic mass<br />
(a larger number of protons) the gravitational pull between the protons and electrons increases, thus increasing the pull<br />
by the protons on the electrons, which causes the electrons to be pulled closer in proximity to the nucleus. This<br />
consequently results in a decrease in size as the electrons are pulled closer and closer to the nucleus.<br />
46