Fuel cells and electrolysers in future energy systems - VBN
Fuel cells and electrolysers in future energy systems - VBN
Fuel cells and electrolysers in future energy systems - VBN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
7 Technology Description<br />
7.1 Introduction<br />
CHP plants have proven to have good <strong>and</strong> efficient abilities to <strong>in</strong>tegrate fluctuat<strong>in</strong>g <strong>energy</strong><br />
sources. The expansion of CHP <strong>systems</strong> is important to <strong>in</strong>crease the overall fuel efficiencies <strong>in</strong><br />
both <strong>energy</strong> <strong>systems</strong> with high amounts of fluctuat<strong>in</strong>g w<strong>in</strong>d power <strong>and</strong> <strong>in</strong> <strong>systems</strong> with low<br />
amounts of fluctuat<strong>in</strong>g w<strong>in</strong>d power. The efficiencies of the CHP plants themselves can be<br />
improved significantly by means of fuel cell technologies. These can improve the overall fuel<br />
efficiency further <strong>and</strong> reduce the environmental impacts connected to the production of <strong>energy</strong>.<br />
In these <strong>cells</strong> chemical <strong>energy</strong> is converted directly <strong>in</strong>to electricity <strong>in</strong>stead of traditional<br />
technologies where the <strong>energy</strong> content <strong>in</strong> fuels is converted <strong>in</strong>to thermal <strong>energy</strong>, then<br />
mechanical <strong>energy</strong> <strong>and</strong> then electricity.<br />
Although several types of fuel <strong>cells</strong> are currently be<strong>in</strong>g developed <strong>and</strong> demonstrated, for large<br />
stationary appliances or micro-CHP one k<strong>in</strong>d of fuel cell is especially promis<strong>in</strong>g because of its<br />
high efficiency <strong>and</strong> fuel flexibility, solid oxide fuel <strong>cells</strong> (SOFC). Other k<strong>in</strong>ds for fuel <strong>cells</strong> are<br />
more suitable for mobile or smaller distributed generation. The most promis<strong>in</strong>g fuel <strong>cells</strong> with<strong>in</strong><br />
these applications are proton exchange membrane fuel <strong>cells</strong> (PEMFC), because of their rather<br />
simple design <strong>and</strong> quick start-up.<br />
For the task of <strong>in</strong>tegration of w<strong>in</strong>d<br />
power <strong>and</strong> other fluctuat<strong>in</strong>g renewable<br />
<strong>energy</strong> sources the different fuel <strong>cells</strong><br />
have different capabilities. <strong>Fuel</strong> <strong>cells</strong>,<br />
like other technologies, cannot be seen<br />
as an isolated improvement, but has to<br />
be assessed with<strong>in</strong> the <strong>energy</strong> system<br />
surround<strong>in</strong>g it. In this section the<br />
advantages <strong>and</strong> disadvantages of us<strong>in</strong>g<br />
fuel <strong>cells</strong> for balanc<strong>in</strong>g fluctuat<strong>in</strong>g<br />
renewable <strong>energy</strong> sources is assessed.<br />
7.2 <strong>Fuel</strong> cell characteristics,<br />
efficiencies <strong>and</strong> applications<br />
All fuel <strong>cells</strong> have <strong>in</strong> common that the<br />
core consists of a cell with an electrolyte<br />
<strong>and</strong> two electrodes, the anode <strong>and</strong> the<br />
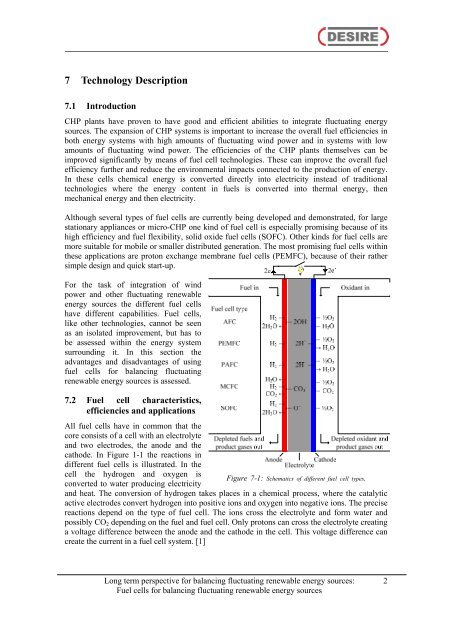
cathode. In Figure 1-1 the reactions <strong>in</strong><br />
different fuel <strong>cells</strong> is illustrated. In the<br />
cell the hydrogen <strong>and</strong> oxygen is<br />
Figure 7-1: Schematics of different fuel cell types.<br />
converted to water produc<strong>in</strong>g electricity<br />
<strong>and</strong> heat. The conversion of hydrogen takes places <strong>in</strong> a chemical process, where the catalytic<br />
active electrodes convert hydrogen <strong>in</strong>to positive ions <strong>and</strong> oxygen <strong>in</strong>to negative ions. The precise<br />
reactions depend on the type of fuel cell. The ions cross the electrolyte <strong>and</strong> form water <strong>and</strong><br />
possibly CO2 depend<strong>in</strong>g on the fuel <strong>and</strong> fuel cell. Only protons can cross the electrolyte creat<strong>in</strong>g<br />
a voltage difference between the anode <strong>and</strong> the cathode <strong>in</strong> the cell. This voltage difference can<br />
create the current <strong>in</strong> a fuel cell system. [1]<br />
Long term perspective for balanc<strong>in</strong>g fluctuat<strong>in</strong>g renewable <strong>energy</strong> sources:<br />
<strong>Fuel</strong> <strong>cells</strong> for balanc<strong>in</strong>g fluctuat<strong>in</strong>g renewable <strong>energy</strong> sources<br />
2