152 153 Intestinal Disease Meeting Berlin 2006 - Dr. Falk Pharma ...
152 153 Intestinal Disease Meeting Berlin 2006 - Dr. Falk Pharma ...
152 153 Intestinal Disease Meeting Berlin 2006 - Dr. Falk Pharma ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
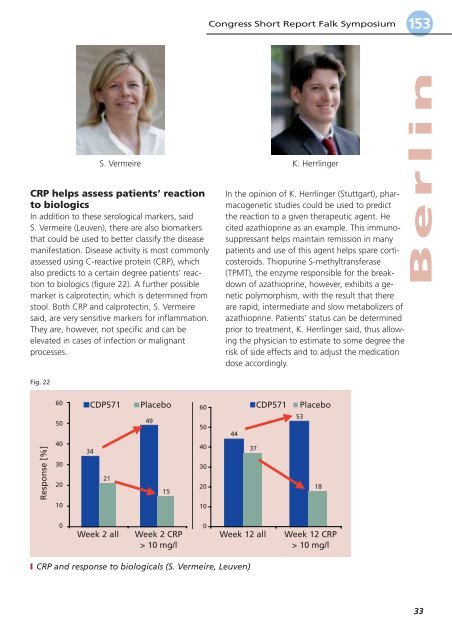
Fig. 22<br />
CDP571<br />
34<br />
21<br />
Placebo<br />
I CRP and response to biologicals (S. Vermeire, Leuven)<br />
49<br />
15<br />
Congress Short Report <strong>Falk</strong> Symposium<br />
S. Vermeire K. Herrlinger<br />
CRP helps assess patients’ reaction<br />
to biologics<br />
In addition to these serological markers, said<br />
S. Vermeire (Leuven), there are also biomarkers<br />
that could be used to better classify the disease<br />
manifestation. <strong>Disease</strong> activity is most commonly<br />
assessed using C-reactive protein (CRP), which<br />
also predicts to a certain degree patients’ reaction<br />
to biologics (figure 22). A further possible<br />
marker is calprotectin, which is determined from<br />
stool. Both CRP and calprotectin, S. Vermeire<br />
said, are very sensitive markers for inflammation.<br />
They are, however, not specific and can be<br />
elevated in cases of infection or malignant<br />
processes.<br />
Response [%]<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
In the opinion of K. Herrlinger (Stuttgart), pharmacogenetic<br />
studies could be used to predict<br />
the reaction to a given therapeutic agent. He<br />
cited azathioprine as an example. This immunosuppressant<br />
helps maintain remission in many<br />
patients and use of this agent helps spare corticosteroids.<br />
Thiopurine S-methyltransferase<br />
(TPMT), the enzyme responsible for the breakdown<br />
of azathioprine, however, exhibits a genetic<br />
polymorphism, with the result that there<br />
are rapid, intermediate and slow metabolizers of<br />
azathioprine. Patients’ status can be determined<br />
prior to treatment, K. Herrlinger said, thus allowing<br />
the physician to estimate to some degree the<br />
risk of side effects and to adjust the medication<br />
dose accordingly.<br />
0<br />
Week 2 all Week 2 CRP Week 12 all Week 12 CRP<br />
> 10 mg/l<br />
> 10 mg/l<br />
44<br />
37<br />
CDP571<br />
53<br />
Placebo<br />
18<br />
<strong>153</strong><br />
33