Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
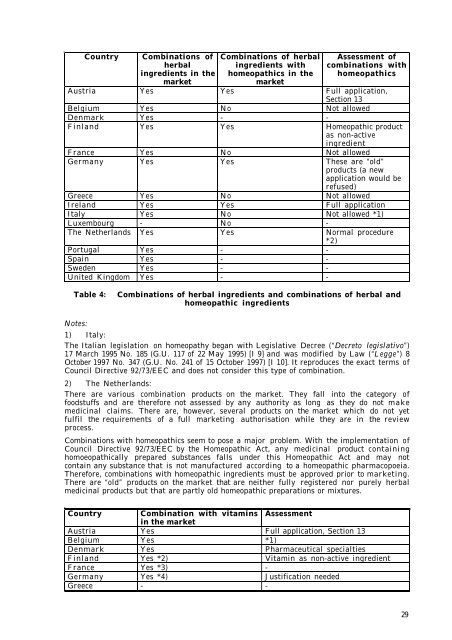
Country Comb<strong>in</strong>ations of<br />
herbal<br />
<strong>in</strong>gredients <strong>in</strong> <strong>the</strong><br />
market<br />
Comb<strong>in</strong>ations of herbal<br />
<strong>in</strong>gredients with<br />
homeopathics <strong>in</strong> <strong>the</strong><br />
market<br />
Assessment of<br />
comb<strong>in</strong>ations with<br />
homeopathics<br />
Austria Yes Yes Full application,<br />
Section 13<br />
Belgium Yes No Not allowed<br />
Denmark Yes - -<br />
F<strong>in</strong>land Yes Yes Homeopathic product<br />
as non-active<br />
<strong>in</strong>gredient<br />
France Yes No Not allowed<br />
Germany Yes Yes These are “old”<br />
<strong>products</strong> (a new<br />
application would be<br />
refused)<br />
Greece Yes No Not allowed<br />
Ireland Yes Yes Full application<br />
Italy Yes No Not allowed *1)<br />
Luxembourg - No -<br />
The Ne<strong>the</strong>rlands Yes Yes Normal procedure<br />
*2)<br />
Portugal Yes - -<br />
Spa<strong>in</strong> Yes - -<br />
Sweden Yes - -<br />
United K<strong>in</strong>gdom Yes - -<br />
Table 4: Comb<strong>in</strong>ations of herbal <strong>in</strong>gredients and comb<strong>in</strong>ations of herbal and<br />
homeopathic <strong>in</strong>gredients<br />
Notes:<br />
1) Italy:<br />
The Italian legislation on homeopathy began with Legislative Decree (“Decreto legislativo”)<br />
17 March 1995 No. 185 (G.U. 117 of 22 May 1995) [I 9] and was modified by Law (“Legge”) 8<br />
October 1997 No. 347 (G.U. No. 241 of 15 October 1997) [I 10]. It reproduces <strong>the</strong> exact terms of<br />
Council Directive 92/73/EEC and does not consider this type of comb<strong>in</strong>ation.<br />
2) The Ne<strong>the</strong>rlands:<br />
There are various comb<strong>in</strong>ation <strong>products</strong> on <strong>the</strong> market. They fall <strong>in</strong>to <strong>the</strong> category of<br />
foodstuffs and are <strong>the</strong>refore not assessed by any authority as long as <strong>the</strong>y do not make<br />
<strong>medic<strong>in</strong>al</strong> claims. There are, however, several <strong>products</strong> on <strong>the</strong> market which do not yet<br />
fulfil <strong>the</strong> requirements of a full market<strong>in</strong>g authorisation while <strong>the</strong>y are <strong>in</strong> <strong>the</strong> review<br />
process.<br />
Comb<strong>in</strong>ations with homeopathics seem to pose a major problem. With <strong>the</strong> implementation of<br />
Council Directive 92/73/EEC by <strong>the</strong> Homeopathic Act, any <strong>medic<strong>in</strong>al</strong> product conta<strong>in</strong><strong>in</strong>g<br />
homoeopathically prepared substances falls under this Homeopathic Act and may not<br />
conta<strong>in</strong> any substance that is not manufactured accord<strong>in</strong>g to a homeopathic pharmacopoeia.<br />
Therefore, comb<strong>in</strong>ations with homeopathic <strong>in</strong>gredients must be approved prior to market<strong>in</strong>g.<br />
There are “old” <strong>products</strong> on <strong>the</strong> market that are nei<strong>the</strong>r fully registered nor purely herbal<br />
<strong>medic<strong>in</strong>al</strong> <strong>products</strong> but that are partly old homeopathic preparations or mixtures.<br />
Country Comb<strong>in</strong>ation with vitam<strong>in</strong>s<br />
<strong>in</strong> <strong>the</strong> market<br />
Assessment<br />
Austria Yes Full application, Section 13<br />
Belgium Yes *1)<br />
Denmark Yes Pharmaceutical specialties<br />
F<strong>in</strong>land Yes *2) Vitam<strong>in</strong> as non-active <strong>in</strong>gredient<br />
France Yes *3) -<br />
Germany Yes *4) Justification needed<br />
Greece - -<br />
29