Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
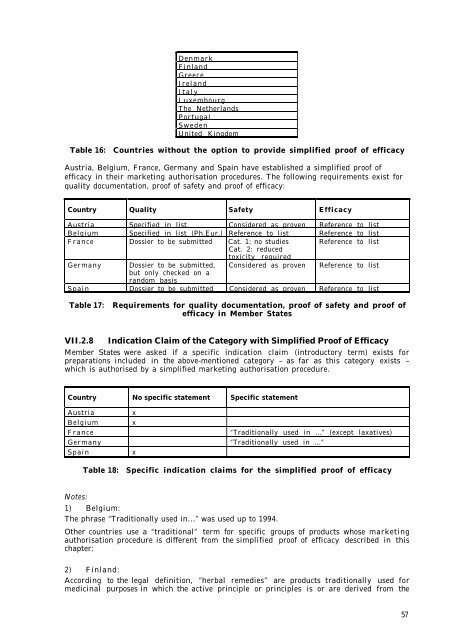
Denmark<br />
F<strong>in</strong>land<br />
Greece<br />
Ireland<br />
Italy<br />
Luxembourg<br />
The Ne<strong>the</strong>rlands<br />
Portugal<br />
Sweden<br />
United K<strong>in</strong>gdom<br />
Table 16: Countries without <strong>the</strong> option to provide simplified proof of efficacy<br />
Austria, Belgium, France, Germany and Spa<strong>in</strong> have established a simplified proof of<br />
efficacy <strong>in</strong> <strong>the</strong>ir market<strong>in</strong>g authorisation procedures. The follow<strong>in</strong>g requirements exist for<br />
quality documentation, proof of safety and proof of efficacy:<br />
Country Quality Safety Efficacy<br />
Austria Specified <strong>in</strong> list Considered as proven Reference to list<br />
Belgium Specified <strong>in</strong> list (Ph.Eur.) Reference to list Reference to list<br />
France Dossier to be submitted Cat. 1: no studies<br />
Cat. 2: reduced<br />
toxicity required<br />
Reference to list<br />
Germany Dossier to be submitted,<br />
but only checked on a<br />
random basis<br />
Considered as proven Reference to list<br />
Spa<strong>in</strong> Dossier to be submitted Considered as proven Reference to list<br />
Table 17: Requirements for quality documentation, proof of safety and proof of<br />
efficacy <strong>in</strong> Member States<br />
VII.2.8 Indication Claim of <strong>the</strong> Category with Simplified Proof of Efficacy<br />
Member States were asked if a specific <strong>in</strong>dication claim (<strong>in</strong>troductory term) exists for<br />
preparations <strong>in</strong>cluded <strong>in</strong> <strong>the</strong> above-mentioned category – as far as this category exists –<br />
which is authorised by a simplified market<strong>in</strong>g authorisation procedure.<br />
Country No specific statement Specific statement<br />
Austria x<br />
Belgium x<br />
France “Traditionally used <strong>in</strong> ...” (except laxatives)<br />
Germany “Traditionally used <strong>in</strong> ...”<br />
Spa<strong>in</strong> x<br />
Table 18: Specific <strong>in</strong>dication claims for <strong>the</strong> simplified proof of efficacy<br />
Notes:<br />
1) Belgium:<br />
The phrase “Traditionally used <strong>in</strong>…” was used up to 1994.<br />
O<strong>the</strong>r countries use a “traditional” term for specific groups of <strong>products</strong> whose market<strong>in</strong>g<br />
authorisation procedure is different from <strong>the</strong> simplified proof of efficacy described <strong>in</strong> this<br />
chapter:<br />
2) F<strong>in</strong>land:<br />
Accord<strong>in</strong>g to <strong>the</strong> legal def<strong>in</strong>ition, “herbal remedies” are <strong>products</strong> traditionally used for<br />
<strong>medic<strong>in</strong>al</strong> purposes <strong>in</strong> which <strong>the</strong> active pr<strong>in</strong>ciple or pr<strong>in</strong>ciples is or are derived from <strong>the</strong><br />
57