Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
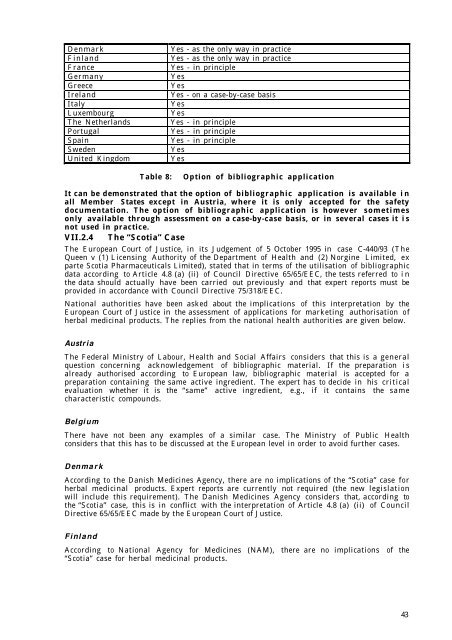
Denmark Yes - as <strong>the</strong> only way <strong>in</strong> practice<br />
F<strong>in</strong>land Yes - as <strong>the</strong> only way <strong>in</strong> practice<br />
France Yes - <strong>in</strong> pr<strong>in</strong>ciple<br />
Germany Yes<br />
Greece Yes<br />
Ireland Yes - on a case-by-case basis<br />
Italy Yes<br />
Luxembourg Yes<br />
The Ne<strong>the</strong>rlands Yes - <strong>in</strong> pr<strong>in</strong>ciple<br />
Portugal Yes - <strong>in</strong> pr<strong>in</strong>ciple<br />
Spa<strong>in</strong> Yes - <strong>in</strong> pr<strong>in</strong>ciple<br />
Sweden Yes<br />
United K<strong>in</strong>gdom Yes<br />
Table 8: Option of bibliographic application<br />
It can be demonstrated that <strong>the</strong> option of bibliographic application is available i n<br />
all Member States except <strong>in</strong> Austria, where it is only accepted for <strong>the</strong> safety<br />
documentation. The option of bibliographic application is however sometimes<br />
only available through assessment on a case-by-case basis, or <strong>in</strong> several cases it i s<br />
not used <strong>in</strong> practice.<br />
VII.2.4 The “Scotia” Case<br />
The <strong>European</strong> Court of Justice, <strong>in</strong> its Judgement of 5 October 1995 <strong>in</strong> case C-440/93 (The<br />
Queen v (1) Licens<strong>in</strong>g Authority of <strong>the</strong> Department of Health and (2) Norg<strong>in</strong>e Limited, ex<br />
parte Scotia Pharmaceuticals Limited), stated that <strong>in</strong> terms of <strong>the</strong> utilisation of bibliographic<br />
data accord<strong>in</strong>g to Article 4.8 (a) (ii) of Council Directive 65/65/EEC, <strong>the</strong> tests referred to i n<br />
<strong>the</strong> data should actually have been carried out previously and that expert reports must be<br />
provided <strong>in</strong> accordance with Council Directive 75/318/EEC.<br />
National authorities have been asked about <strong>the</strong> implications of this <strong>in</strong>terpretation by <strong>the</strong><br />
<strong>European</strong> Court of Justice <strong>in</strong> <strong>the</strong> assessment of applications for market<strong>in</strong>g authorisation of<br />
herbal <strong>medic<strong>in</strong>al</strong> <strong>products</strong>. The replies from <strong>the</strong> national health authorities are given below.<br />
Austria<br />
The Federal M<strong>in</strong>istry of Labour, Health and Social Affairs considers that this is a general<br />
question concern<strong>in</strong>g acknowledgement of bibliographic material. If <strong>the</strong> preparation is<br />
already authorised accord<strong>in</strong>g to <strong>European</strong> law, bibliographic material is accepted for a<br />
preparation conta<strong>in</strong><strong>in</strong>g <strong>the</strong> same active <strong>in</strong>gredient. The expert has to decide <strong>in</strong> his critical<br />
evaluation whe<strong>the</strong>r it is <strong>the</strong> “same” active <strong>in</strong>gredient, e.g., if it conta<strong>in</strong>s <strong>the</strong> same<br />
characteristic compounds.<br />
Belgium<br />
There have not been any examples of a similar case. The M<strong>in</strong>istry of Public Health<br />
considers that this has to be discussed at <strong>the</strong> <strong>European</strong> level <strong>in</strong> order to avoid fur<strong>the</strong>r cases.<br />
Denmark<br />
Accord<strong>in</strong>g to <strong>the</strong> Danish Medic<strong>in</strong>es Agency, <strong>the</strong>re are no implications of <strong>the</strong> “Scotia” case for<br />
herbal <strong>medic<strong>in</strong>al</strong> <strong>products</strong>. Expert reports are currently not required (<strong>the</strong> new legislation<br />
will <strong>in</strong>clude this requirement). The Danish Medic<strong>in</strong>es Agency considers that, accord<strong>in</strong>g to<br />
<strong>the</strong> “Scotia” case, this is <strong>in</strong> conflict with <strong>the</strong> <strong>in</strong>terpretation of Article 4.8 (a) (ii) of Council<br />
Directive 65/65/EEC made by <strong>the</strong> <strong>European</strong> Court of Justice.<br />
F<strong>in</strong>land<br />
Accord<strong>in</strong>g to National Agency for Medic<strong>in</strong>es (NAM), <strong>the</strong>re are no implications of <strong>the</strong><br />
“Scotia” case for herbal <strong>medic<strong>in</strong>al</strong> <strong>products</strong>.<br />
43