Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
Herbal medicinal products in the European Union - AESGP
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
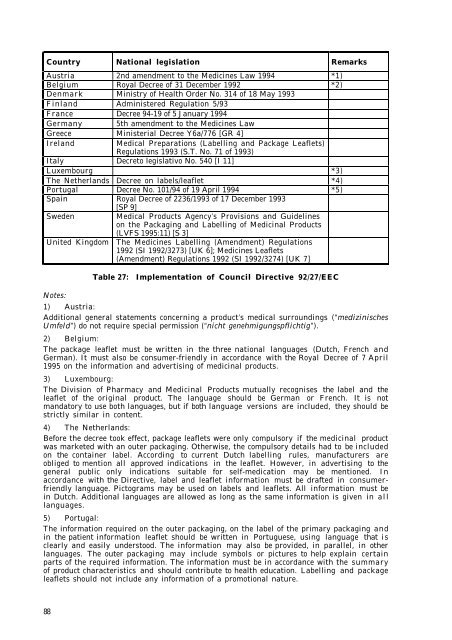
Country National legislation Remarks<br />
Austria 2nd amendment to <strong>the</strong> Medic<strong>in</strong>es Law 1994 *1)<br />
Belgium Royal Decree of 31 December 1992 *2)<br />
Denmark M<strong>in</strong>istry of Health Order No. 314 of 18 May 1993<br />
F<strong>in</strong>land Adm<strong>in</strong>istered Regulation 5/93<br />
France Decree 94-19 of 5 January 1994<br />
Germany 5th amendment to <strong>the</strong> Medic<strong>in</strong>es Law<br />
Greece M<strong>in</strong>isterial Decree Y6a/776 [GR 4]<br />
Ireland Medical Preparations (Labell<strong>in</strong>g and Package Leaflets)<br />
Regulations 1993 (S.T. No. 71 of 1993)<br />
Italy Decreto legislativo No. 540 [I 11]<br />
Luxembourg *3)<br />
The Ne<strong>the</strong>rlands Decree on labels/leaflet *4)<br />
Portugal Decree No. 101/94 of 19 April 1994 *5)<br />
Spa<strong>in</strong> Royal Decree of 2236/1993 of 17 December 1993<br />
[SP 9]<br />
Sweden Medical Products Agency’s Provisions and Guidel<strong>in</strong>es<br />
on <strong>the</strong> Packag<strong>in</strong>g and Labell<strong>in</strong>g of Medic<strong>in</strong>al Products<br />
(LVFS 1995:11) [S 3]<br />
United K<strong>in</strong>gdom The Medic<strong>in</strong>es Labell<strong>in</strong>g (Amendment) Regulations<br />
1992 (SI 1992/3273) [UK 6]; Medic<strong>in</strong>es Leaflets<br />
(Amendment) Regulations 1992 (SI 1992/3274) [UK 7]<br />
88<br />
Table 27: Implementation of Council Directive 92/27/EEC<br />
Notes:<br />
1) Austria:<br />
Additional general statements concern<strong>in</strong>g a product’s medical surround<strong>in</strong>gs (“mediz<strong>in</strong>isches<br />
Umfeld”) do not require special permission (“nicht genehmigungspflichtig”).<br />
2) Belgium:<br />
The package leaflet must be written <strong>in</strong> <strong>the</strong> three national languages (Dutch, French and<br />
German). It must also be consumer-friendly <strong>in</strong> accordance with <strong>the</strong> Royal Decree of 7 April<br />
1995 on <strong>the</strong> <strong>in</strong>formation and advertis<strong>in</strong>g of <strong>medic<strong>in</strong>al</strong> <strong>products</strong>.<br />
3) Luxembourg:<br />
The Division of Pharmacy and Medic<strong>in</strong>al Products mutually recognises <strong>the</strong> label and <strong>the</strong><br />
leaflet of <strong>the</strong> orig<strong>in</strong>al product. The language should be German or French. It is not<br />
mandatory to use both languages, but if both language versions are <strong>in</strong>cluded, <strong>the</strong>y should be<br />
strictly similar <strong>in</strong> content.<br />
4) The Ne<strong>the</strong>rlands:<br />
Before <strong>the</strong> decree took effect, package leaflets were only compulsory if <strong>the</strong> <strong>medic<strong>in</strong>al</strong> product<br />
was marketed with an outer packag<strong>in</strong>g. O<strong>the</strong>rwise, <strong>the</strong> compulsory details had to be <strong>in</strong>cluded<br />
on <strong>the</strong> conta<strong>in</strong>er label. Accord<strong>in</strong>g to current Dutch labell<strong>in</strong>g rules, manufacturers are<br />
obliged to mention all approved <strong>in</strong>dications <strong>in</strong> <strong>the</strong> leaflet. However, <strong>in</strong> advertis<strong>in</strong>g to <strong>the</strong><br />
general public only <strong>in</strong>dications suitable for self-medication may be mentioned. In<br />
accordance with <strong>the</strong> Directive, label and leaflet <strong>in</strong>formation must be drafted <strong>in</strong> consumerfriendly<br />
language. Pictograms may be used on labels and leaflets. All <strong>in</strong>formation must be<br />
<strong>in</strong> Dutch. Additional languages are allowed as long as <strong>the</strong> same <strong>in</strong>formation is given <strong>in</strong> all<br />
languages.<br />
5) Portugal:<br />
The <strong>in</strong>formation required on <strong>the</strong> outer packag<strong>in</strong>g, on <strong>the</strong> label of <strong>the</strong> primary packag<strong>in</strong>g and<br />
<strong>in</strong> <strong>the</strong> patient <strong>in</strong>formation leaflet should be written <strong>in</strong> Portuguese, us<strong>in</strong>g language that i s<br />
clearly and easily understood. The <strong>in</strong>formation may also be provided, <strong>in</strong> parallel, <strong>in</strong> o<strong>the</strong>r<br />
languages. The outer packag<strong>in</strong>g may <strong>in</strong>clude symbols or pictures to help expla<strong>in</strong> certa<strong>in</strong><br />
parts of <strong>the</strong> required <strong>in</strong>formation. The <strong>in</strong>formation must be <strong>in</strong> accordance with <strong>the</strong> summary<br />
of product characteristics and should contribute to health education. Labell<strong>in</strong>g and package<br />
leaflets should not <strong>in</strong>clude any <strong>in</strong>formation of a promotional nature.