ehr onc final certification - Department of Health Care Services
ehr onc final certification - Department of Health Care Services
ehr onc final certification - Department of Health Care Services
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ability <strong>of</strong> CCD and CCR to support the inclusion <strong>of</strong> the discharge summary and the<br />
principle expressed by CMS that we specify a minimum set <strong>of</strong> information in the adopted<br />
<strong>certification</strong> criterion, we believe that in this instance it is appropriate to exclude<br />
discharge summary from the <strong>certification</strong> criterion.<br />
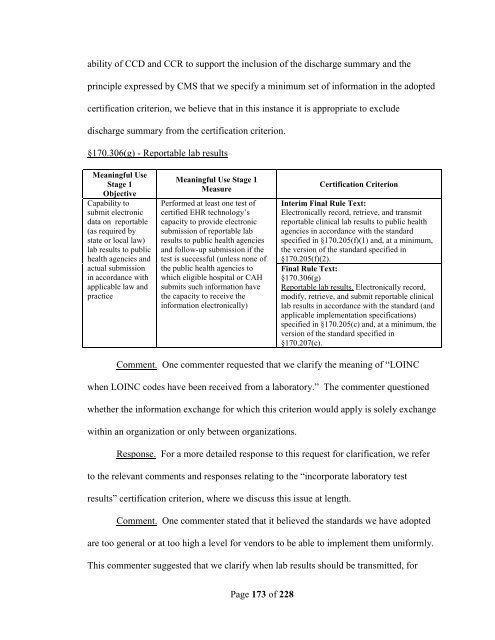
§170.306(g) - Reportable lab results<br />
Meaningful Use<br />
Stage 1<br />
Objective<br />
Capability to<br />
submit electronic<br />
data on reportable<br />
(as required by<br />
state or local law)<br />
lab results to public<br />
health agencies and<br />
actual submission<br />
in accordance with<br />
applicable law and<br />
practice<br />
Meaningful Use Stage 1<br />
Measure<br />
Performed at least one test <strong>of</strong><br />
certified EHR technology’s<br />
capacity to provide electronic<br />
submission <strong>of</strong> reportable lab<br />
results to public health agencies<br />
and follow-up submission if the<br />
test is successful (unless none <strong>of</strong><br />
the public health agencies to<br />
which eligible hospital or CAH<br />
submits such information have<br />
the capacity to receive the<br />
information electronically)<br />
Page 173 <strong>of</strong> 228<br />
Certification Criterion<br />
Interim Final Rule Text:<br />
Electronically record, retrieve, and transmit<br />
reportable clinical lab results to public health<br />
agencies in accordance with the standard<br />
specified in §170.205(f)(1) and, at a minimum,<br />
the version <strong>of</strong> the standard specified in<br />
§170.205(f)(2).<br />
Final Rule Text:<br />
§170.306(g)<br />
Reportable lab results. Electronically record,<br />
modify, retrieve, and submit reportable clinical<br />
lab results in accordance with the standard (and<br />
applicable implementation specifications)<br />
specified in §170.205(c) and, at a minimum, the<br />
version <strong>of</strong> the standard specified in<br />
§170.207(c).<br />
Comment. One commenter requested that we clarify the meaning <strong>of</strong> “LOINC<br />
when LOINC codes have been received from a laboratory.” The commenter questioned<br />
whether the information exchange for which this criterion would apply is solely exchange<br />
within an organization or only between organizations.<br />
Response. For a more detailed response to this request for clarification, we refer<br />
to the relevant comments and responses relating to the “incorporate laboratory test<br />
results” <strong>certification</strong> criterion, where we discuss this issue at length.<br />
Comment. One commenter stated that it believed the standards we have adopted<br />
are too general or at too high a level for vendors to be able to implement them uniformly.<br />
This commenter suggested that we clarify when lab results should be transmitted, for