Spectroscopy in a Suitcase - Royal Society of Chemistry
Spectroscopy in a Suitcase - Royal Society of Chemistry
Spectroscopy in a Suitcase - Royal Society of Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ULTRAVIOLET - VISIBLE SPECTROSCOPY (UV)<br />
EXERCISE 2 - REACTION OF BLUE FOOD DYE WITH BLEACH 5<br />
MATERIALS<br />
Chemicals<br />
• FD&C Blue #1 Erioglauc<strong>in</strong>e (Cas# 3844-45-9)<br />
Mw = 792.86 g/mol (C 37 H 34 N 2 O 9 S 3 •2Na)<br />
• Stock solution: 0.0104 g <strong>in</strong> 50 cm 3 ;<br />
c=2.263x10 -4 mol dm -3<br />
• 2 cm 3 <strong>of</strong> stock solution <strong>in</strong> 50 cm 3 ;<br />
c=9.05x10 -6 mol dm -3 to give an absorbance<br />
<strong>of</strong> ~0.7 at lmax. (15 ml per student/pair)<br />
• De-ionised water (15 ml per student/pair)<br />
• Bleach solution – Hypochlorite 6% w/v<br />
(15 ml per student/pair)<br />
Apparatus (Per student or pair)<br />
• 3 x 25 ml beaker<br />
• 3 x 2 ml plastic disposable syr<strong>in</strong>ge without needle<br />
(Pipettes can be used <strong>in</strong>stead but syr<strong>in</strong>ge quicker for<br />
tak<strong>in</strong>g k<strong>in</strong>etics measurements and to aid mix<strong>in</strong>g)<br />
• 2 x disposable plastic cuvettes and stoppers<br />
• Tissues<br />
Instrument<br />
• UV-visible Spectrometer (<strong>in</strong>tegral pr<strong>in</strong>ter and paper)<br />
Initial scan 400 – 700 nm then set to s<strong>in</strong>gle<br />
wavelength approximately 628 nm<br />
• Laptop (optional)<br />
• Pr<strong>in</strong>ter (optional)<br />
• Connection cables x 2 (optional)<br />
Set up for laptop and pr<strong>in</strong>ter use:<br />
• Connect UV-vis to laptop via left hand front<br />
USB port (Com 5)<br />
• Connect pr<strong>in</strong>ter to any USB port<br />
• From spectrometer menu Select pr<strong>in</strong>ter /<br />
auto pr<strong>in</strong>t on / Computer USB / OK<br />
• Open PVC program, set auto pr<strong>in</strong>t to on or <strong>of</strong>f<br />
depend<strong>in</strong>g on requirements.<br />
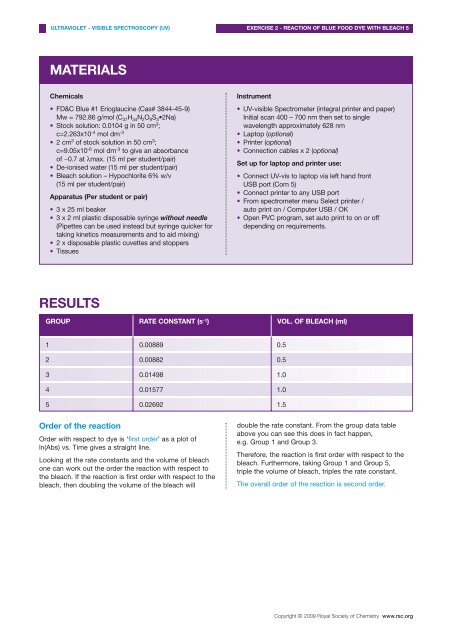
RESULTS<br />
GROUP RATE CONSTANT (s -1 ) VOL. OF BLEACH (ml)<br />
1 0.00889 0.5<br />
2 0.00882 0.5<br />
3 0.01498 1.0<br />
4 0.01577 1.0<br />
5 0.02692 1.5<br />
Order <strong>of</strong> the reaction<br />
Order with respect to dye is ‘first order’ as a plot <strong>of</strong><br />
ln(Abs) vs. Time gives a straight l<strong>in</strong>e.<br />
Look<strong>in</strong>g at the rate constants and the volume <strong>of</strong> bleach<br />
one can work out the order the reaction with respect to<br />
the bleach. If the reaction is first order with respect to the<br />
bleach, then doubl<strong>in</strong>g the volume <strong>of</strong> the bleach will<br />
double the rate constant. From the group data table<br />
above you can see this does <strong>in</strong> fact happen,<br />
e.g. Group 1 and Group 3.<br />
Therefore, the reaction is first order with respect to the<br />
bleach. Furthermore, tak<strong>in</strong>g Group 1 and Group 5,<br />
triple the volume <strong>of</strong> bleach, triples the rate constant.<br />
The overall order <strong>of</strong> the reaction is second order.<br />
Copyright © 2009 <strong>Royal</strong> <strong>Society</strong> <strong>of</strong> <strong>Chemistry</strong> www.rsc.org