Spectroscopy in a Suitcase - Royal Society of Chemistry
Spectroscopy in a Suitcase - Royal Society of Chemistry
Spectroscopy in a Suitcase - Royal Society of Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ULTRAVIOLET - VISIBLE SPECTROSCOPY (UV)<br />
EXERCISE 4 - INVESTIGATING TRANSITION METAL COMPLEXES 5<br />
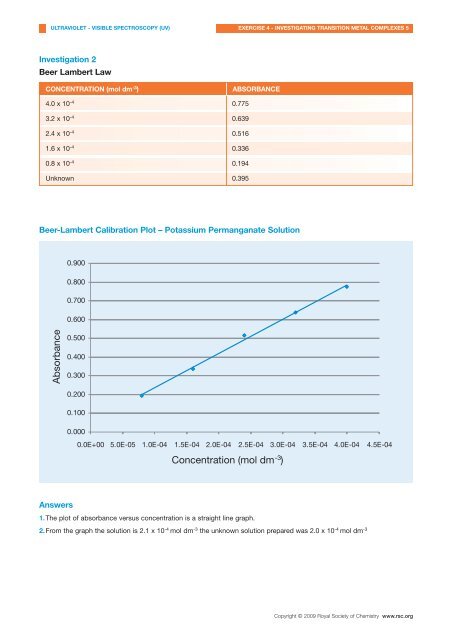
Investigation 2<br />
Beer Lambert Law<br />
CONCENTRATION (mol dm -3 )<br />
ABSORBANCE<br />
4.0 x 10 -4 0.775<br />
3.2 x 10 -4 0.639<br />
2.4 x 10 -4 0.516<br />
1.6 x 10 -4 0.336<br />
0.8 x 10 -4 0.194<br />
Unknown 0.395<br />
Beer-Lambert Calibration Plot – Potassium Permanganate Solution<br />
0.900<br />
0.800<br />
0.700<br />
0.600<br />
Absorbance<br />
0.500<br />
0.400<br />
0.300<br />
0.200<br />
0.100<br />
0.000<br />
0.0E+00 5.0E-05 1.0E-04 1.5E-04 2.0E-04 2.5E-04 3.0E-04 3.5E-04 4.0E-04 4.5E-04<br />
Concentration (mol dm -3 )<br />
Answers<br />
1.The plot <strong>of</strong> absorbance versus concentration is a straight l<strong>in</strong>e graph.<br />
2.From the graph the solution is 2.1 x 10 -4 mol dm -3 the unknown solution prepared was 2.0 x 10 -4 mol dm -3<br />
Copyright © 2009 <strong>Royal</strong> <strong>Society</strong> <strong>of</strong> <strong>Chemistry</strong> www.rsc.org