BABSc, B.Com & BCA Questions _III - Nalanda Open University
BABSc, B.Com & BCA Questions _III - Nalanda Open University
BABSc, B.Com & BCA Questions _III - Nalanda Open University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
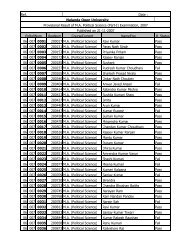
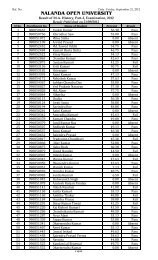
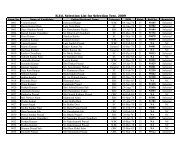
<strong>Nalanda</strong> <strong>Open</strong> <strong>University</strong><br />
Annual Exam-2010,<br />
Bachelor of Science (Chemistry) Hons, Part-<strong>III</strong><br />
Paper-VI<br />
Time: 3 Hrs Full Marks: 75<br />
Answer any FIVE questions. All questions are of equal marks.<br />
1. (a) Give an outline of how SchrÖdinger Wave equation is used to arrive at<br />
the shapes of orbital via radial and angular distribution curves.<br />
(b) Show that the energy of a particle in one-dimension potential box is<br />
quantized.<br />
2. Explain the terms. Probability and radial probability of electron in an atom.<br />
Draw radial probability distribution curves for s and p orbital.<br />
3. Draw and explain the molecular orbital diagram of H + 2 molecule ion, He 2 and<br />
Li 2 molecule. Evaluate their bond orders and comment on magnetic behaviour of<br />
each one.<br />
4. Write critical short notes on any THREE of the following.<br />
(a) Electromagnetic wave and matter wave.<br />
(b) Bond strength of N 2 and F 2 molecule in term of MOT.<br />
(c) Anti-bonding and non-bonding orbital.<br />
(d) Possible orientations for the orbital in the f sub-shell.<br />
5. How the solvents have been classified? <strong>Com</strong>pare the merits of liquid sulphur<br />
dioxide and water as solvents. Under what conditions sulphur dioxide acts as a<br />
neutral solvent?<br />
6. Name the ores of platinum. Give flow-sheet diagram for the extraction of<br />
platinum from Sudbury ore. Discuss important properties, types of platinum<br />
metals and their use. Why is platinum called noble metal?<br />
7. Explain how the atomic orbital combine to form bonding and anti-bonding<br />
molecular orbital? What are the limitations of such combination?<br />
8. (a) By giving suitable examples, differentiate a symmetry element from<br />
symmetry operation.<br />
(b) Show that NH 3 and BF 3 are placed in different symmetry point groups.<br />
9. Explain in brief diamagnetic and paramagnetic. Write macroscopic and<br />
microscopic causes of such magnetic behaviour.<br />
10. Elaborate role of sodium, potassium and magnesium in plants and animals.<br />
κ κ κ