Heat
heat-story
heat-story
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Observed Climate Changes and Impacts<br />
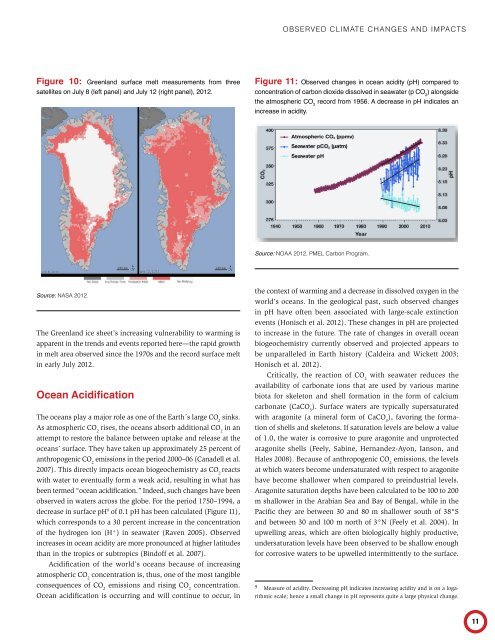
Figure 10: Greenland surface melt measurements from three<br />
satellites on July 8 (left panel) and July 12 (right panel), 2012.<br />
Figure 11: Observed changes in ocean acidity (pH) compared to<br />
concentration of carbon dioxide dissolved in seawater (p CO 2<br />
) alongside<br />
the atmospheric CO 2<br />
record from 1956. A decrease in pH indicates an<br />
increase in acidity.<br />
Source: NOAA 2012, PMEL Carbon Program.<br />
Source: NASA 2012.<br />
The Greenland ice sheet’s increasing vulnerability to warming is<br />
apparent in the trends and events reported here—the rapid growth<br />
in melt area observed since the 1970s and the record surface melt<br />
in early July 2012.<br />
Ocean Acidification<br />
The oceans play a major role as one of the Earth´s large CO 2<br />
sinks.<br />
As atmospheric CO 2<br />
rises, the oceans absorb additional CO 2<br />
in an<br />
attempt to restore the balance between uptake and release at the<br />
oceans’ surface. They have taken up approximately 25 percent of<br />
anthropogenic CO 2<br />
emissions in the period 2000–06 (Canadell et al.<br />
2007). This directly impacts ocean biogeochemistry as CO 2<br />
reacts<br />
with water to eventually form a weak acid, resulting in what has<br />
been termed “ocean acidification.” Indeed, such changes have been<br />
observed in waters across the globe. For the period 1750–1994, a<br />
decrease in surface pH 9 of 0.1 pH has been calculated (Figure 11),<br />
which corresponds to a 30 percent increase in the concentration<br />
of the hydrogen ion (H + ) in seawater (Raven 2005). Observed<br />
increases in ocean acidity are more pronounced at higher latitudes<br />
than in the tropics or subtropics (Bindoff et al. 2007).<br />
Acidification of the world’s oceans because of increasing<br />
atmospheric CO 2<br />
concentration is, thus, one of the most tangible<br />
consequences of CO 2<br />
emissions and rising CO 2<br />
concentration.<br />
Ocean acidification is occurring and will continue to occur, in<br />
the context of warming and a decrease in dissolved oxygen in the<br />
world’s oceans. In the geological past, such observed changes<br />
in pH have often been associated with large-scale extinction<br />
events (Honisch et al. 2012). These changes in pH are projected<br />
to increase in the future. The rate of changes in overall ocean<br />
biogeochemistry currently observed and projected appears to<br />
be unparalleled in Earth history (Caldeira and Wickett 2003;<br />
Honisch et al. 2012).<br />
Critically, the reaction of CO 2<br />
with seawater reduces the<br />
availability of carbonate ions that are used by various marine<br />
biota for skeleton and shell formation in the form of calcium<br />
carbonate (CaCO 3<br />
). Surface waters are typically supersaturated<br />
with aragonite (a mineral form of CaCO 3<br />
), favoring the formation<br />
of shells and skeletons. If saturation levels are below a value<br />
of 1.0, the water is corrosive to pure aragonite and unprotected<br />
aragonite shells (Feely, Sabine, Hernandez-Ayon, Ianson, and<br />
Hales 2008). Because of anthropogenic CO 2<br />
emissions, the levels<br />
at which waters become undersaturated with respect to aragonite<br />
have become shallower when compared to preindustrial levels.<br />
Aragonite saturation depths have been calculated to be 100 to 200<br />
m shallower in the Arabian Sea and Bay of Bengal, while in the<br />
Pacific they are between 30 and 80 m shallower south of 38°S<br />
and between 30 and 100 m north of 3°N (Feely et al. 2004). In<br />
upwelling areas, which are often biologically highly productive,<br />
undersaturation levels have been observed to be shallow enough<br />
for corrosive waters to be upwelled intermittently to the surface.<br />
9 Measure of acidity. Decreasing pH indicates increasing acidity and is on a logarithmic<br />
scale; hence a small change in pH represents quite a large physical change.<br />
11