Screening for cancer: are biomarkers of value?
Screening for cancer: are biomarkers of value?
Screening for cancer: are biomarkers of value?
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
– February/March 2011 6 Tumour markers<br />
<strong>Screening</strong> <strong>for</strong> <strong>cancer</strong>:<br />
<strong>are</strong> <strong>biomarkers</strong> <strong>of</strong> <strong>value</strong>?<br />
Intuitively, the measurement <strong>of</strong> <strong>biomarkers</strong> <strong>for</strong> <strong>cancer</strong> screening has<br />
great appeal. This article reviews the current status <strong>of</strong> the <strong>biomarkers</strong><br />
most widely studied in <strong>cancer</strong> screening, but concludes that their use<br />
to date has been disappointing in reducing mortality from <strong>cancer</strong>,<br />
especially in asymptomatic populations. Their main utility in screening<br />
is likely to be in high-risk populations, where the prevalence <strong>of</strong> <strong>cancer</strong><br />
is high. An example is the use <strong>of</strong> human chorionic gonadotropin<br />
<strong>for</strong> screening <strong>for</strong> gestational trophoblastic neoplasia in patients<br />
who have had a previous diagnosis <strong>of</strong> a hydatidi<strong>for</strong>m mole.<br />
by Pr<strong>of</strong>. Michael J Duffy<br />
<strong>Screening</strong> <strong>for</strong> <strong>cancer</strong> has great intuitive appeal,<br />
since it is widely believed that the early detection<br />
<strong>of</strong> malignancy followed by the initiation<br />
<strong>of</strong> treatment results in improved outcome.<br />
Consequently, population-based screening<br />
<strong>for</strong> different types <strong>of</strong> <strong>cancer</strong> is now available<br />
in many countries, including mammography<br />
<strong>for</strong> breast <strong>cancer</strong> in women over 50 years <strong>of</strong><br />
age, the PAP smear <strong>for</strong> cervical <strong>cancer</strong> and<br />
either colonoscopy or faecal occult blood<br />
testing (FOBT) <strong>for</strong> colorectal <strong>cancer</strong>.<br />
Comp<strong>are</strong>d to procedures such as mammography,<br />
colonoscopy and smear testing, the<br />
measurement <strong>of</strong> <strong>biomarkers</strong> is considerably<br />
simpler and more convenient [1]. However,<br />
as previously pointed out by Dr Sturgeon [3],<br />
<strong>biomarkers</strong> generally have low sensitivities<br />
and low specificities <strong>for</strong> early malignancy.<br />
These disadvantages, when combined with<br />
the low prevalence <strong>of</strong> <strong>cancer</strong>s in a healthy<br />
population, greatly limit the use <strong>of</strong> <strong>biomarkers</strong><br />
in screening programmes, especially<br />
when used alone. Despite these disadvantages,<br />
several <strong>biomarkers</strong> have either undergone<br />
or <strong>are</strong> currently undergoing investigation<br />
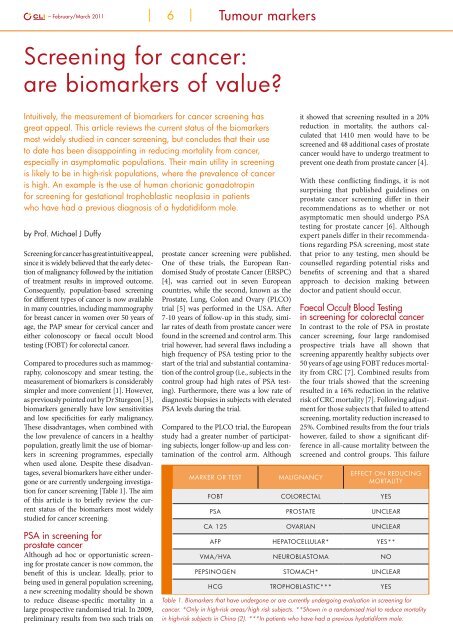
<strong>for</strong> <strong>cancer</strong> screening [Table 1]. The aim<br />
<strong>of</strong> this article is to briefly review the current<br />
status <strong>of</strong> the <strong>biomarkers</strong> most widely<br />
studied <strong>for</strong> <strong>cancer</strong> screening.<br />
PSA in screening <strong>for</strong><br />
prostate <strong>cancer</strong><br />
Although ad hoc or opportunistic screening<br />
<strong>for</strong> prostate <strong>cancer</strong> is now common, the<br />
benefit <strong>of</strong> this is unclear. Ideally, prior to<br />
being used in general population screening,<br />
a new screening modality should be shown<br />
to reduce disease-specific mortality in a<br />
large prospective randomised trial. In 2009,<br />
preliminary results from two such trials on<br />
prostate <strong>cancer</strong> screening were published.<br />
One <strong>of</strong> these trials, the European Randomised<br />
Study <strong>of</strong> prostate Cancer (ERSPC)<br />
[4], was carried out in seven European<br />
countries, while the second, known as the<br />
Prostate, Lung, Colon and Ovary (PLCO)<br />
trial [5] was per<strong>for</strong>med in the USA. After<br />
7-10 years <strong>of</strong> follow-up in this study, similar<br />
rates <strong>of</strong> death from prostate <strong>cancer</strong> were<br />
found in the screened and control arm. This<br />
trial however, had several flaws including a<br />
high frequency <strong>of</strong> PSA testing prior to the<br />
start <strong>of</strong> the trial and substantial contamination<br />
<strong>of</strong> the control group (i.e., subjects in the<br />
control group had high rates <strong>of</strong> PSA testing).<br />
Furthermore, there was a low rate <strong>of</strong><br />
diagnostic biopsies in subjects with elevated<br />
PSA levels during the trial.<br />
Comp<strong>are</strong>d to the PLCO trial, the European<br />
study had a greater number <strong>of</strong> participating<br />
subjects, longer follow-up and less contamination<br />
<strong>of</strong> the control arm. Although<br />
Marker or test<br />
it showed that screening resulted in a 20%<br />
reduction in mortality, the authors calculated<br />
that 1410 men would have to be<br />
screened and 48 additional cases <strong>of</strong> prostate<br />
<strong>cancer</strong> would have to undergo treatment to<br />
prevent one death from prostate <strong>cancer</strong> [4].<br />
With these conflicting findings, it is not<br />
surprising that published guidelines on<br />
prostate <strong>cancer</strong> screening differ in their<br />
recommendations as to whether or not<br />
asymptomatic men should undergo PSA<br />
testing <strong>for</strong> prostate <strong>cancer</strong> [6]. Although<br />
expert panels differ in their recommendations<br />
regarding PSA screening, most state<br />
that prior to any testing, men should be<br />
counselled regarding potential risks and<br />
benefits <strong>of</strong> screening and that a sh<strong>are</strong>d<br />
approach to decision making between<br />
doctor and patient should occur.<br />
Faecal Occult Blood Testing<br />
in screening <strong>for</strong> colorectal <strong>cancer</strong><br />
In contrast to the role <strong>of</strong> PSA in prostate<br />
<strong>cancer</strong> screening, four large randomised<br />
prospective trials have all shown that<br />
screening app<strong>are</strong>ntly healthy subjects over<br />
50 years <strong>of</strong> age using FOBT reduces mortality<br />
from CRC [7]. Combined results from<br />
the four trials showed that the screening<br />
resulted in a 16% reduction in the relative<br />
risk <strong>of</strong> CRC mortality [7]. Following adjustment<br />
<strong>for</strong> those subjects that failed to attend<br />
screening, mortality reduction increased to<br />
25%. Combined results from the four trials<br />
however, failed to show a significant difference<br />
in all-cause mortality between the<br />
screened and control groups. This failure<br />
Malignancy<br />
Effect on reducing<br />
mortality<br />
FOBT Colorectal Yes<br />
PSA Prostate Unclear<br />
CA 125 Ovarian Unclear<br />
AFP Hepatocellular* Yes**<br />
VMA/HVA Neuroblastoma No<br />
Pepsinogen Stomach* Unclear<br />
HCG Trophoblastic*** Yes<br />
Table 1. Biomarkers that have undergone or <strong>are</strong> currently undergoing evaluation in screening <strong>for</strong><br />
<strong>cancer</strong>. *Only in high-risk <strong>are</strong>as/high risk subjects. **Shown in a randomised trial to reduce mortality<br />
in high-risk subjects in China (2). ***In patients who have had a previous hydatidi<strong>for</strong>m mole.