Real time PCR - European Pharmaceutical Review
Real time PCR - European Pharmaceutical Review
Real time PCR - European Pharmaceutical Review
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
THERMAL ANALYSIS ISSUE 2009<br />
Calorimetry as a<br />
Process Analytical Tool<br />
for micronising<br />
pharmaceuticals<br />
Simon Gaisford, Lecturer in Pharmaceutics, University of London<br />
Drug delivery via the pulmonary route is becoming increasingly popular, but brings a unique set of<br />
formulation challenges. In particular the small particle sizes (between 2-5 μm) required to facilitate<br />
deposition in the lung frequently mean that size-reduction steps (such as milling) are required<br />
during processing of constituents (usually the active principal) prior to formulation.<br />
Typically either ball mills or air-jet<br />
mills are employed to achieve<br />
the desired reduction in particle<br />
size. In the former case ceramic or<br />
metal balls are placed in a container<br />
with the sample and whole apparatus<br />
is rotated; the size, weight and number<br />
of balls can be varied as can the<br />
number of revolutions per minute<br />
and the total milling <strong>time</strong>. In the latter<br />
case compressed air is used to<br />
agitate particles, causing size reduction<br />
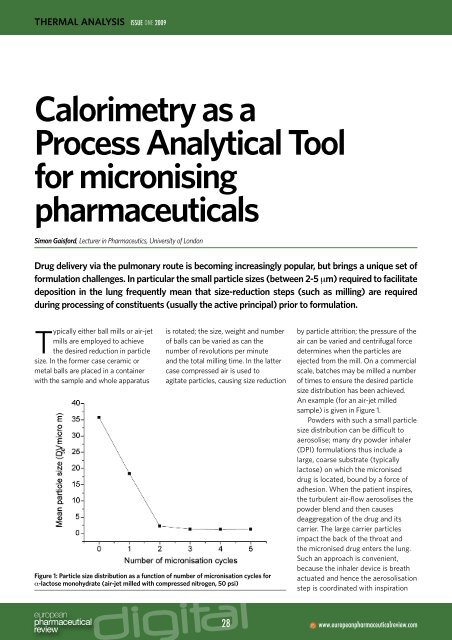
Figure 1: Particle size distribution as a function of number of micronisation cycles for<br />
α-lactose monohydrate (air-jet milled with compressed nitrogen, 50 psi)<br />
by particle attrition; the pressure of the<br />
air can be varied and centrifugal force<br />
determines when the particles are<br />
ejected from the mill. On a commercial<br />
scale, batches may be milled a number<br />
of <strong>time</strong>s to ensure the desired particle<br />
size distribution has been achieved.<br />
An example (for an air-jet milled<br />
sample) is given in Figure 1.<br />
Powders with such a small particle<br />
size distribution can be difficult to<br />
aerosolise; many dry powder inhaler<br />
(DPI) formulations thus include a<br />
large, coarse substrate (typically<br />
lactose) on which the micronised<br />
drug is located, bound by a force of<br />
adhesion. When the patient inspires,<br />
the turbulent air-flow aerosolises the<br />
powder blend and then causes<br />
deaggregation of the drug and its<br />
carrier. The large carrier particles<br />
impact the back of the throat and<br />
the micronised drug enters the lung.<br />
Such an approach is convenient,<br />
because the inhaler device is breath<br />
actuated and hence the aerosolisation<br />
step is coordinated with inspiration<br />
28<br />
www.europeanpharmaceuticalreview.com