Real time PCR - European Pharmaceutical Review
Real time PCR - European Pharmaceutical Review
Real time PCR - European Pharmaceutical Review
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SUBSCRIBE<br />
ISSUE<br />
2009 THERMAL ANALYSIS<br />
(a common problem with pressurised<br />
metered dose inhalers (pMDI)).<br />
As has been discussed in the<br />
literature 1 , the processes involved in<br />
particle size reduction during milling<br />
are complex. The mechanical forces<br />
imparted to a crystalline material<br />
during milling result initially in a<br />
reduction in particle size, as the<br />
material fractures along natural fault<br />
lines and crystal defects. Eventually<br />
there comes a point at which the bulk<br />
material can no longer fracture and<br />
maximum particle size reduction has<br />
been achieved. Post this point,<br />
mechanical forces are still being<br />
applied and must be absorbed and<br />
dissipated by the sample; these<br />
processes result initially in production<br />
of surface dislocations and eventually<br />
generation of amorphous regions; so<br />
called process-induced disorder.<br />
As has been well documented<br />
elsewhere 2,3 , the amount of<br />
amorphous material formed in this<br />
way is usually small (of the order of a<br />
few percent w/w); however, the nature<br />
of the force applied (impaction)<br />
means that this amorphous material<br />
must necessarily be located on the<br />
surface of the milled material and,<br />
hence, although the milled material is<br />
predominantly crystalline it behaves<br />
as if it were entirely amorphous.<br />
Newell at al 4 published some inverse<br />
phase gas chromatography (IGC) data<br />
(see Table 1) that convey this point. It<br />
is inevitable that this amorphous<br />
material will crystallise, probably over<br />
a relatively short <strong>time</strong>frame as it is<br />
located on a crystalline substrate that<br />
can act as a seed. Crystallisation of<br />
the surface may have disastrous<br />
consequences for the function of the<br />
product 5 , primarily because the force<br />
of adhesion between drug and carrier<br />
will change, altering the balance of<br />
forces that is so important in<br />
modulating product performance from<br />
batch-to-batch and within one batch<br />
over <strong>time</strong>. As such, it is imperative to<br />
understand the mechanisms and<br />
kinetics of crystallisation, such that its<br />
effects can be ameliorated. Often this<br />
means conditioning (under humidity<br />
or some other suitable plasticising<br />
vapour) the material post micronising<br />
to force crystallisation.<br />
If it is true that particle size<br />
reduction occurs before, and not<br />
concomitantly with, generation of<br />
process induced disorder then it is<br />
apparent that it would be possible to<br />
micronise a sample without<br />
generating any amorphous material.<br />
This would remove the need for<br />
downstream conditioning and ensure<br />
consistent product performance. It is<br />
not possible to optimise milling by<br />
following particle size reduction<br />
alone, since there is no particle sizing<br />
technique that can simultaneously<br />
detect formation of amorphous<br />
content. Returning to the data shown<br />
in Figure 1 (page 28), it is tempting to<br />
set up a process involving four to five<br />
micronising steps, since this seems<br />
to ensure a consistent particle size<br />
distribution; such an approach<br />
does nothing to report the generation<br />
of process induced disorder and<br />
can lead to the batch-to-batch<br />
variability in product performance<br />
noted above.<br />
Here, we show that following the<br />
micronising process with an<br />
additional technique, isothermal<br />
microcalorimetry, affords extra data<br />
that allows optimisation of the<br />
micronising <strong>time</strong>. In addition, the data<br />
allow the hypothesis that particle size<br />
reduction occurs before the<br />
generation of process induced<br />
disorder to be tested.<br />
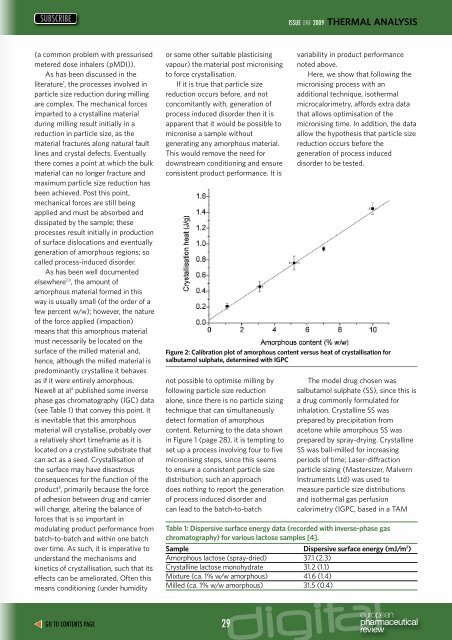
Figure 2: Calibration plot of amorphous content versus heat of crystallisation for<br />
salbutamol sulphate, determined with IGPC<br />
The model drug chosen was<br />
salbutamol sulphate (SS), since this is<br />
a drug commonly formulated for<br />
inhalation. Crystalline SS was<br />
prepared by precipitation from<br />
acetone while amorphous SS was<br />
prepared by spray-drying. Crystalline<br />
SS was ball-milled for increasing<br />
periods of <strong>time</strong>; Laser-diffraction<br />
particle sizing (Mastersizer, Malvern<br />
Instruments Ltd) was used to<br />
measure particle size distributions<br />
and isothermal gas perfusion<br />
calorimetry (IGPC, based in a TAM<br />
Table 1: Dispersive surface energy data (recorded with inverse-phase gas<br />
chromatography) for various lactose samples [4].<br />
Sample Dispersive surface energy (mJ/m 2 )<br />
Amorphous lactose (spray-dried) 37.1 (2.3)<br />
Crystalline lactose monohydrate 31.2 (1.1)<br />
Mixture (ca. 1% w/w amorphous) 41.6 (1.4)<br />
Milled (ca. 1% w/w amorphous) 31.5 (0.4)<br />
GO TO CONTENTS PAGE<br />
29