Monograph - Metrohm
Monograph - Metrohm
Monograph - Metrohm
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Practical thermometric titrimetry 21<br />
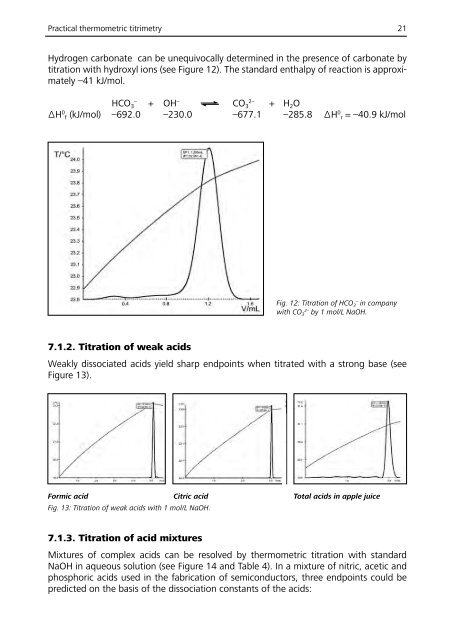
Hydrogen carbonate can be unequivocally determined in the presence of carbonate by<br />
titration with hydroxyl ions (see Figure 12). The standard enthalpy of reaction is approximately<br />
–41 kJ/mol.<br />
–<br />
HCO 3 + OH – 2–<br />
CO 3 + H 2 O<br />
ΔH 0 f (kJ/mol) –692.0 –230.0 –677.1 –285.8 ΔH 0 r = –40.9 kJ/mol<br />
Fig. 12: Titration of HCO 3<br />
–<br />
in company<br />
with CO 3<br />
2–<br />
by 1 mol/L NaOH.<br />
7.1.2. Titration of weak acids<br />
Weakly dissociated acids yield sharp endpoints when titrated with a strong base (see<br />
Figure 13).<br />
Formic acid Citric acid Total acids in apple juice<br />
Fig. 13: Titration of weak acids with 1 mol/L NaOH.<br />
7.1.3. Titration of acid mixtures<br />
Mixtures of complex acids can be resolved by thermometric titration with standard<br />
NaOH in aqueous solution (see Figure 14 and Table 4). In a mixture of nitric, acetic and<br />
phosphoric acids used in the fabrication of semiconductors, three endpoints could be<br />
predicted on the basis of the dissociation constants of the acids: