Monograph - Metrohm
Monograph - Metrohm
Monograph - Metrohm
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Practical thermometric titrimetry 31<br />
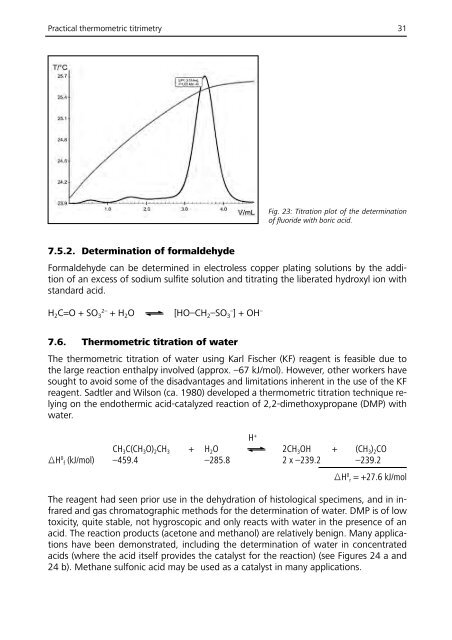
Fig. 23: Titration plot of the determination<br />
of fluoride with boric acid.<br />
7.5.2. Determination of formaldehyde<br />
Formaldehyde can be determined in electroless copper plating solutions by the addition<br />
of an excess of sodium sulfite solution and titrating the liberated hydroxyl ion with<br />
standard acid.<br />
H 2 C=O + SO 3<br />
2–<br />
+ H 2 O [HO–CH 2 –SO 3– ] + OH –<br />
7.6. Thermometric titration of water<br />
The thermometric titration of water using Karl Fischer (KF) reagent is feasible due to<br />
the large reaction enthalpy involved (approx. –67 kJ/mol). However, other workers have<br />
sought to avoid some of the disadvantages and limitations inherent in the use of the KF<br />
reagent. Sadtler and Wilson (ca. 1980) developed a thermometric titration technique relying<br />
on the endothermic acid-catalyzed reaction of 2,2-dimethoxypropane (DMP) with<br />
water.<br />
H +<br />
CH 3 C(CH 3 O) 2 CH 3 + H 2 O 2CH 3 OH + (CH 3 ) 2 CO<br />
ΔH 0 f (kJ/mol) –459.4 –285.8 2 x –239.2 –239.2<br />
ΔH 0 r = +27.6 kJ/mol<br />
The reagent had seen prior use in the dehydration of histological specimens, and in infrared<br />
and gas chromatographic methods for the determination of water. DMP is of low<br />
toxicity, quite stable, not hygroscopic and only reacts with water in the presence of an<br />
acid. The reaction products (acetone and methanol) are relatively benign. Many applications<br />
have been demonstrated, including the determination of water in concentrated<br />
acids (where the acid itself provides the catalyst for the reaction) (see Figures 24 a and<br />
24 b). Methane sulfonic acid may be used as a catalyst in many applications.