Monograph - Metrohm
Monograph - Metrohm
Monograph - Metrohm
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
30 Practical thermometric titrimetry<br />
Thermometric titrimetry offers an opportunity to observe the influence of micelles on<br />
the conduct of the determination. To avoid the influence of micelle-related phenomena<br />
on the outcome of the titration, it is sometimes necessary to add a suitable solvent such<br />
as acetonitrile.<br />
7.4.6. Titration of non-ionic surfactants<br />
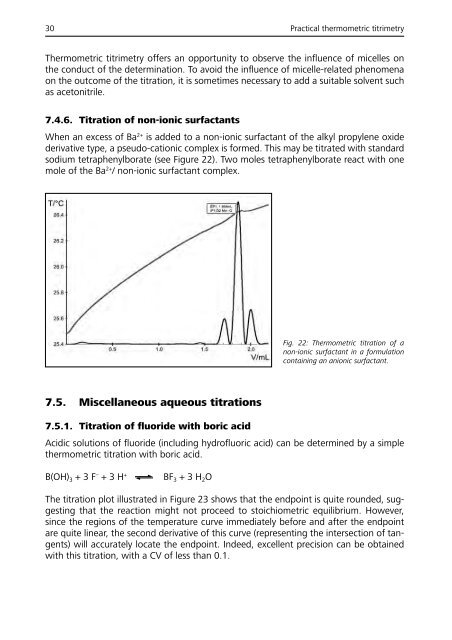
When an excess of Ba 2+ is added to a non-ionic surfactant of the alkyl propylene oxide<br />
derivative type, a pseudo-cationic complex is formed. This may be titrated with standard<br />
sodium tetraphenylborate (see Figure 22). Two moles tetraphenylborate react with one<br />
mole of the Ba 2+ / non-ionic surfactant complex.<br />
Fig. 22: Thermometric titration of a<br />
non-ionic surfactant in a formulation<br />
containing an anionic surfactant.<br />
7.5. Miscellaneous aqueous titrations<br />
7.5.1. Titration of fluoride with boric acid<br />
Acidic solutions of fluoride (including hydrofluoric acid) can be determined by a simple<br />
thermometric titration with boric acid.<br />
B(OH) 3 + 3 F – + 3 H +<br />
BF 3 + 3 H 2 O<br />
The titration plot illustrated in Figure 23 shows that the endpoint is quite rounded, suggesting<br />
that the reaction might not proceed to stoichiometric equilibrium. However,<br />
since the regions of the temperature curve immediately before and after the endpoint<br />
are quite linear, the second derivative of this curve (representing the intersection of tangents)<br />
will accurately locate the endpoint. Indeed, excellent precision can be obtained<br />
with this titration, with a CV of less than 0.1.