synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Synthesis <strong>of</strong> Potential 5-HT 6 Receptor Lig<strong>and</strong>s<br />
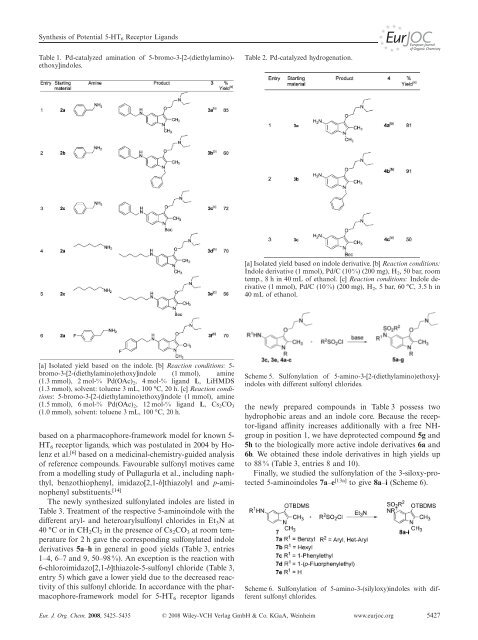
Table 1. Pd-catalyzed amination <strong>of</strong> 5-bromo-3-[2-(diethylamino)ethoxy]<strong>indoles</strong>.<br />
[a] Isolated yield based on the indole. [b] Reaction conditions: 5bromo-3-[2-(diethylamino)ethoxy]indole<br />
(1 mmol), amine<br />
(1.3 mmol), 2 mol-% Pd(OAc) 2, 4 mol-% lig<strong>and</strong> L, LiHMDS<br />
(1.3 mmol), solvent: toluene 3 mL, 100 °C, 20 h. [c] Reaction conditions:<br />
5-bromo-3-[2-(diethylamino)ethoxy]indole (1 mmol), amine<br />
(1.5 mmol), 6 mol-% Pd(OAc) 2, 12 mol-% lig<strong>and</strong> L, Cs 2CO 3<br />
(1.0 mmol), solvent: toluene 3 mL, 100 °C, 20 h.<br />
based on a pharmacophore-framework model for known 5-<br />
HT 6 receptor lig<strong>and</strong>s, which was postulated in 2004 by Holenz<br />
et al. [6] based on a medicinal-chemistry-guided analysis<br />
<strong>of</strong> reference compounds. Favourable sulfonyl motives came<br />
from a modelling study <strong>of</strong> Pullagurla et al., including naphthyl,<br />
benzothiophenyl, imidazo[2,1-b]thiazolyl <strong>and</strong> p-aminophenyl<br />
substituents. [14]<br />
The newly synthesized sulfonylated <strong>indoles</strong> are listed in<br />
Table 3. Treatment <strong>of</strong> the respective 5-aminoindole with the<br />
different aryl- <strong>and</strong> heteroarylsulfonyl chlorides in Et 3Nat<br />
40 °C or in CH 2Cl 2 in the presence <strong>of</strong> Cs 2CO 3 at room temperature<br />
for 2 h gave the corresponding sulfonylated indole<br />
derivatives 5a–h in general in good yields (Table 3, entries<br />
1–4, 6–7 <strong>and</strong> 9, 50–98%). An exception is the reaction with<br />
6-chloroimidazo[2,1-b]thiazole-5-sulfonyl chloride (Table 3,<br />
entry 5) which gave a lower yield due to the decreased reactivity<br />
<strong>of</strong> this sulfonyl chloride. In accordance with the pharmacophore-framework<br />
model for 5-HT 6 receptor lig<strong>and</strong>s<br />
Table 2. Pd-catalyzed hydrogenation.<br />
[a] Isolated yield based on indole derivative. [b] Reaction conditions:<br />
Indole derivative (1 mmol), Pd/C (10%) (200 mg), H 2, 50 bar, room<br />
temp., 8 h in 40 mL <strong>of</strong> ethanol. [c] Reaction conditions: Indole derivative<br />
(1 mmol), Pd/C (10%) (200 mg), H 2, 5 bar, 60 °C, 3.5 h in<br />
40 mL <strong>of</strong> ethanol.<br />
Scheme 5. Sulfonylation <strong>of</strong> 5-amino-3-[2-(diethylamino)ethoxy]<strong>indoles</strong><br />
with different sulfonyl chlorides.<br />
the newly prepared compounds in Table 3 possess two<br />
hydrophobic areas <strong>and</strong> an indole core. Because the receptor-lig<strong>and</strong><br />
affinity increases additionally with a free NHgroup<br />
in position 1, we have deprotected compound 5g <strong>and</strong><br />
5h to the <strong>biologically</strong> more <strong>active</strong> indole derivatives 6a <strong>and</strong><br />
6b. We obtained these indole derivatives in high yields up<br />
to 88% (Table 3, entries 8 <strong>and</strong> 10).<br />
Finally, we studied the sulfonylation <strong>of</strong> the 3-siloxy-protected<br />
5-amino<strong>indoles</strong> 7a–e [13a] to give 8a–i (Scheme 6).<br />
Scheme 6. Sulfonylation <strong>of</strong> 5-amino-3-(silyloxy)<strong>indoles</strong> with different<br />
sulfonyl chlorides.<br />
Eur. J. Org. Chem. 2008, 5425–5435 © 2008 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.eurjoc.org 5427