synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1 Palladium-catalyzed Coupling Reactions <strong>of</strong> Indoles 36<br />
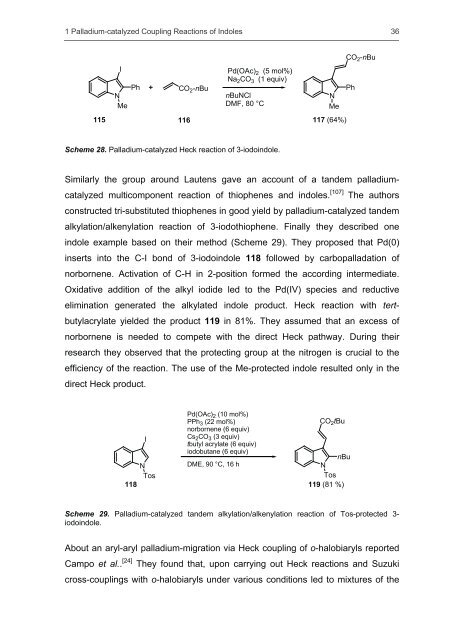
I<br />
N<br />
Me<br />
Ph<br />
CO 2-nBu<br />
Pd(OAc) 2 (5 mol%)<br />
Na 2CO 3 (1 equiv)<br />
nBuNCl<br />
DMF, 80 °C<br />
N<br />
Me<br />
115 116 117 (64%)<br />
Scheme 28. Palladium-catalyzed Heck reaction <strong>of</strong> 3-iodoindole.<br />
CO 2-nBu<br />
Similarly the group around Lautens gave an account <strong>of</strong> a t<strong>and</strong>em palladiumcatalyzed<br />
multicomponent reaction <strong>of</strong> thiophenes <strong>and</strong> <strong>indoles</strong>. [107] The authors<br />
constructed tri-substituted thiophenes in good yield by palladium-catalyzed t<strong>and</strong>em<br />
alkylation/alkenylation reaction <strong>of</strong> 3-iodothiophene. Finally they described one<br />
indole example based on their method (Scheme 29). They proposed that Pd(0)<br />
inserts into the C-I bond <strong>of</strong> 3-iodoindole 118 followed by carbopalladation <strong>of</strong><br />
norbornene. Activation <strong>of</strong> C-H in 2-position formed the according intermediate.<br />
Oxidative addition <strong>of</strong> the alkyl iodide led to the Pd(IV) species <strong>and</strong> reductive<br />
elimination generated the alkylated indole product. Heck reaction with tertbutylacrylate<br />
yielded the product 119 in 81%. They assumed that an excess <strong>of</strong><br />
norbornene is needed to compete with the direct Heck pathway. During their<br />
research they observed that the protecting group at the nitrogen is crucial to the<br />
efficiency <strong>of</strong> the reaction. The use <strong>of</strong> the Me-protected indole resulted only in the<br />
direct Heck product.<br />
Pd(OAc) 2 (10 mol%)<br />
PPh3 (22 mol%)<br />
norbornene (6 equiv)<br />
CO2tBu I<br />
Cs2CO3 (3 equiv)<br />
tbutyl acrylate (6 equiv)<br />
iodobutane (6 equiv)<br />
nBu<br />
N<br />
DME, 90 °C, 16 h N<br />
Tos<br />
Tos<br />
118 119 (81 %)<br />
Scheme 29. Palladium-catalyzed t<strong>and</strong>em alkylation/alkenylation reaction <strong>of</strong> Tos-protected 3iodoindole.<br />
About an aryl-aryl palladium-migration via Heck coupling <strong>of</strong> o-halobiaryls reported<br />
Campo et al.. [24] They found that, upon carrying out Heck reactions <strong>and</strong> Suzuki<br />
cross-couplings with o-halobiaryls under various conditions led to mixtures <strong>of</strong> the<br />
Ph